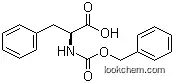
1161-13-3Relevant articles and documents
Selecting optimal conditions for Alcalase CLEA-OM for synthesis of dipeptides in organic media
Vossenberg,Beeftink,Nuijens,Cohen Stuart,Tramper
, p. 43 - 49 (2012)
In protease-catalyzed peptide synthesis, the availability of water is essential, as a compromise must be made between on the one hand the overall enzymatic activity and, on the other hand, the rate of product synthesis. Water is essential for enzyme activity, but at the same time causes hydrolytic side reactions. We studied the coupling of the carbamoylmethyl ester of N-protected phenylalanine and phenylalanine amide in tetrahydrofuran catalyzed by Alcalase CLEA-OM at a range of water activity (aw) values, including the coupling in the presence of molecular sieves (i.e. at very low aw values). The hydrolytic side reaction (in the present system only the hydrolysis of substrate occurs) was found to dominate above an aw value of about 0.2. To prevent hydrolysis, the presence of molecular sieves was found to be necessary.
Aryl hydrazides as linkers for solid phase synthesis which are cleavable under mild oxidative conditions
Millington, Christopher R.,Quarrell, Rachel,Lowe, Gordon
, p. 7201 - 7204 (1998)
We have developed an acid/base stable aryl hydrazide linker which is readily coupled to solid phase resins. Cleavage is specific and facile, requiring a copper (II) catalyst, base and a nucleophile to proceed. The conditions are compatible with all 20 proteinogenic amino acids and quantitative cleavage is achieved within 2 hours at 20°C to give peptides with C-terminal acid, amide or ester functionalities. Aryl hydrazides also offer scope as simple 'traceless' linkers.
Peptiligase, an Enzyme for Efficient Chemoenzymatic Peptide Synthesis and Cyclization in Water
Toplak, Ana,Nuijens, Timo,Quaedflieg, Peter J. L. M.,Wu, Bian,Janssen, Dick B.
, p. 2140 - 2147 (2016)
We describe a novel, organic cosolvent-stable and cation-independent engineered enzyme for peptide coupling reactions. The enzyme is a variant of a stable calcium-independent mutant of subtilisin BPN′, with the catalytic Ser212 mutated to Cys and Pro216 converted to Ala. The enzyme, called peptiligase, catalyzes exceptionally efficient peptide coupling in water with a surprisingly high synthesis over hydrolysis (S/H) ratio. The S/H ratio of the peptide ligation reaction is correlated to the length of the peptide substrate and proved to be >100 for the synthesis of a 13-mer peptide, which corresponds to >99% conversion to the ligated peptide product and 1% hydrolytic side-reaction. Furthermore, peptiligase does not require a particular recognition motif resulting in a broadly applicable and traceless peptide ligation technology. Peptiligase is very robust, easy to produce in Bacillus subtilis, and its purification is straightforward. It shows good activity and stability in the presence of organic cosolvents and chelating or denaturing agents, enabling the ligation of poorly soluble (hydrophobic) or folded peptides. This enzyme could be useful for the (industrial) synthesis of diverse (pharmaceutical) peptides. In addition, peptiligase is able to efficiently catalyze head-to-tail peptide cyclization reactions. (Figure presented.) .
A transient non-covalent hydrogel by a supramolecular gelator with dynamic covalent bonds
Mondal, Sahabaj,Haldar, Debasish
, p. 4773 - 4779 (2021)
Nature operates out of equilibrium, which needs a continuous input of energy. However, on the removal of the energy source, the system returns to its thermodynamically stable building blocks, resulting in an aggregation-to-nonaggregation transition. The control of this system is governed by kinetics. Herein, we have alleviated that system using a supramolecular gelator with dynamic covalent bonds. In the aqueous solution of benzyloxycarbonyl-l-phenylalanine (ZF), equilibrium self-assembly and gelation take place at 4 mg mL?1; however, on the addition of 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC), non-equilibrium hydrogels are formed at 2 mg mL?1due to anhydride formation and self-assembly. However, with time, the hydrolysis of anhydride results in a gel-to-sol transition. The dynamic covalent bond formation and rupture have programmed the dissipative, transient supramolecular hydrogels from ZF with a high degree of control over the self-assembly lifetime. The system is not fully dissipative as it does not spontaneously oscillate between two states, but needs fuel to undergo a transition. The refueling of the system with EDC helps to access multiple cycles.
Can self-assembled hydrogels composed of aromatic amino acid derivatives function as drug delivery carriers?
Tiwari, Priyanka,Rajagopalan, Ramanathan,Moin, Mohammad,Soni, Rohit,Trivedi, Piyush,DuttKonar, Anita
, p. 308 - 315 (2016)
Low molecular weight hydrogelators (LMOHGs) have attracted recent attention due to their diversified applications. In an attempt to artificially imitate their importance in the design of drug delivery carriers, we have synthesized two simple N-terminally protected aromatic amino-acid derivatives that form efficient stable hydrogels at room temperature. The gelation properties of the hydrogels have been thoroughly investigated using various techniques and their strength has been determined by rheological studies. In order to explore the efficacy of the hydrogels as tools for drug delivery, we have developed hydrogel nanoparticles (HNPs) using a surfactant and high-speed homogenization approach. Interestingly, our hydrogel nanoparticles display good entrapment efficiency and release kinetics of the model drug 5-fluoro uracil from the hydrogel matrix. Our experimental results reveal that hydrogel II displays slightly higher efficiency as a drug delivery carrier, which may be due to the presence of an aromatic ring in the backbone in comparison to hydrogel I. This increased strength may be attributed to the increase in π-π interactions when the aromatic residue is present in the backbone. Therefore the nanoparticles generated from hydrogel II may have better hydrogen bonding abilities with drugs in comparison to hydrogel I; thus resulting in a slightly slower release of drug from the hydrogel matrix. This fact may shed some light on the candidature of our hydrogels as future carriers for drug delivery. However, further studies to evaluate the candidature of these novel types of aromatic amino acid hydrogel nanoparticles for nano-medical applications are under investigation.
STEREOSELECTIVE MICELLAR CATALYSIS IN THE HYDROLYSIS OF ENANTIOMERIC ESTERS BY DIPEPTIDE DERIVATIVES CONTAINING HISTIDINE RESIDUE
Ihara, Yasuji,Kunikiyo, Noriko,Kunimasa, Tomoko,Nango, Mamoru,Kuroki, Nobuhiko
, p. 667 - 670 (1981)
The catalytic hydrolysis of enantiomeric substrates is examined using optically active dipeptide derivatives containing histidine residue in the presence of CTABr micelles.A very high stereoselectivity, kc(L)/kc(D), of 12.2 is observed in the reaction with the enantiomers of p-nitrophenyl N-methoxycarbonylphenylalanate (MocPheONp) and N-(benzyloxycarbonyl-L-leucyl)-L-histidine (ZLeuHis).
Substrate specificity of an actively assembling amyloid catalyst
Heier, Jason L.,Mikolajczak, Dorian J.,B?ttcher, Christoph,Koksch, Beate
, (2017)
In the presence of Zn2+, the catalytic, amyloid-forming peptide Ac-IHIHIQI-NH2, was found to exhibit enhanced selectivity for hydrophobic p-nitrophenyl ester substrates while in the process of self-assembly. As opposed to the substrate p-nitrophenyl acetate, which was more effectively hydrolyzed with Ac-IHIHIQI-NH2 in its fully fibrillar state, the hydrophobic substrate Z-L-Phe-ONp was converted with a second-order rate constant more than 11-times greater when the catalyst was actively assembling. Under such conditions, Z-L-Phe-ONp hydrolysis proceeded at a greater velocity than the more hydrophilic and otherwise more labile ester Boc-L-Asn-ONp. When assembling, the catalyst also showed increased selectivity for the L-enantiomer of Z-Phe-ONp. These findings suggest the occurrence of increased interactions of hydrophobic moieties of the substrate with exposed hydrophobic surfaces of the assembling peptides and present valuable features for future de novo design consideration.
Bottom-Up Construction of an Adaptive Enzymatic Reaction Network
Helwig, Britta,van Sluijs, Bob,Pogodaev, Aleksandr A.,Postma, Sjoerd G. J.,Huck, Wilhelm T. S.
, p. 14065 - 14069 (2018)
The reproduction of emergent behaviors in nature using reaction networks is an important objective in synthetic biology and systems chemistry. Herein, the first experimental realization of an enzymatic reaction network capable of an adaptive response is reported. The design is based on the dual activity of trypsin, which activates chymotrypsin while at the same time generating a fluorescent output from a fluorogenic substrate. Once activated, chymotrypsin counteracts the trypsin output by competing for the fluorogenic substrate and producing a non-fluorescent output. It is demonstrated that this network produces a transient fluorescent output under out-of-equilibrium conditions while the input signal persists. Importantly, in agreement with mathematical simulations, we show that optimization of the pulse-like response is an inherent trade-off between maximum amplitude and lowest residual fluorescence.
Aqueous Solutions Containing Amino Acids and Peptides. Part 17. --Pairwise Enthalpic Coefficients for the Interaction of N-Acetyl-L-Phenylalaninamide with some N-Acetylamino Acid Amines at 25 deg C
Blackburn, G. Michael,Kent, Hilary E.,Lilley, Terence H.
, p. 2207 - 2214 (1985)
Enthalpies of dilution of N-acetyl-L-phenylalaninamide and equimolal solutions of this with N-acetylglycinamide, N-acetyl-L-alaninamide, N-acetyl- L-valinamide and N-acetyl-L-leucinamide have been determined using microcalorimetry.The results obtained were used to calculate the pairwise enthalpic coefficients for both like-like (homotactic) and like-unlike (heterotactic) solute interactions.These were then used with a group-additivity approach, which works well for these systems, to obtain information on the interaction of defined groups with each other.
Cobalt-Catalyzed Deprotection of Allyl Carboxylic Esters Induced by Hydrogen Atom Transfer
Li, Nan,Gui, Yizhen,Chu, Mengqi,You, Mengdi,Qiu, Xiaohan,Liu, Hejia,Wang, Shiang,Deng, Meng,Ji, Baoming
supporting information, p. 8460 - 8464 (2021/11/13)
A brief, efficient method has been developed for the removal of the allyl protecting group from allyl carboxylic esters using a Co(II)/TBHP/(Me2SiH)2O catalytic system. This facile strategy displays excellent chemoselectivity, functional group tolerance, and high yields. This transformation probably occurs through the hydrogen atom transfer process, and a Co(III)-six-membered cyclic intermediate is recommended.