99011-02-6 Usage
Uses
Used in Pharmaceutical Industry:
Imiquimod is used as a topical treatment for genital warts caused by human papillomavirus (HPV), as an immunomodulator, and as a caspase 3 activator with antiviral and anti-tumor activity. It is also used to cure actinic keratosis on the face and scalp.
Used in Antiviral Applications:
Imiquimod is used as an antiviral agent, inducing cytokines such as interferon-α (IFN-α), tumor necrosis factor-γ (TNF-γ), interleukins (IL-1α, IL-1β, IL-6, IL-8, IL-10), and granulocyte-macrophage colony-stimulating factor (GM-CSF), which help in the immune response against viral infections.
Used in Anti-tumor Applications:
Imiquimod is used as an anti-tumor agent, displaying activity against basal cell carcinoma and potentially other types of cancer. It modulates the immune response and induces apoptosis in tumor cells.
Used in Immune Response Modification:
Imiquimod is used as an immune response modifier, activating the immune system to fight against abnormal skin growth and potentially other diseases. It is thought to interact with a kinase modulating the transduction pathway leading to cytokine genes, although the exact cells responsible for the response have not been determined.
Drug for treatment of Genital warts
Imiquimod is a kind of imidozoquinoline amine-class interleukin agonist developed by the 3M Pharmaceuticals Company (US), belonging to the drug for the treatment of genital warts. Upon being applied to the mice skin, it can induce the cytokinesis and produce α-interferon, tumor necrosis factor and many kinds of interleukins. Clinically it can be used as the immunomodulators in the treatment of adult genital and perianal warts with convenient application, well tolerance and unique mechanism of action which makes it be the first-choice drug for treatment of genital warts. In addition, there are reports that imiquimod can be used for treating viral skin diseases including common warts, flat warts, molluscum and herpes simplex virus. It can also be effective in treating basal cell carcinoma, Bowen's disease, Bowen papulosis, actinic keratosis, and skin tumors such as cutaneous T-cell tumor and Kaposi's sarcoma. It may also be effective in treating vitiligo and alopecia areata.
Genital wart is the infective diseases of the human herpes virus (HPV) infection and is a major public health threat. Currently, the infection of the genital wart in the United States is the most common viral infective sexually transmitted diseases and one of the most common sexually transmitted diseases. The infections mostly occur in sexually active people, especially for people of 20 to 24 year-olds and women in pregnancy, and prone to happen at the warm and most mucosal sites of body. Application of immunosuppressive agents or infection with the patients of human immunodeficiency virus is prone to cause severe clinical signs of infection.
Clinical Study
In an unpublished report, 209 cases of patients of genital or perianal warts have been subjected to a double-blind trial with both this product and vehicles being used for three times per week for continuous 16 weeks. The results have shown that, 33 cases in 46 cases of women (72%), 21 cases in 63 cases of male(33%) get cured with the total wart cure rate being 50%, while only 11% (20% of women and 5% male) in the vehicles group get cured. There have been patients who get cured within treatment of only 4 weeks. The average healing time is twelve weeks for men and eight weeks for women. In the following 12 weeks, there were 39 cases in 54 cases that got no recurrence any more.
Mechanism of action
Imiquimod itself has no direct effect on the virus itself, but instead through stimulating the body for producing cytokines and stimulating immune response to eliminate the wart tissue and reduce the viral erosion.
Both in vivo and in vitro studies have suggested that imiquimod can stimulate the mice, rats, rabbits, guinea pigs, monkeys and humans for producing cytokine. Racial cross cell culture tests have shown that the main responsive cells are monocytes/macrophages; cultured human peripheral mononuclear cells (PBMC) which can produce several subtypes of IFN-α, TNF-α, IL-1,6,6 , 10,12, MIP-1 and MCP-1; the human single cell THP-1 will respond only when being pre-subject to γ-IFN treatment and produce TNF-α, IL-1,6,8, but do not produce IFN-α; performing the same treatment on the fresh mice PBMC can generate the same cytokine as human PBMC; senescence macrophages and alveolar macrophages produce the same cytokines, the mouse RAW264.7 and J774 macrophages family can produce TNF-α and IL-6 without producing IFN-α. Through intracellular test, we can confirm that imiquimod can induce IFN-α and TNF-α.
Through putting the human fibroblasts and keratinocytes with the mixture of imiquimod or poly-inosine: poly-cytidine for incubation of 24 hours, people has found that the mRNA of keratinocytes IL-6 and IL-8 (no TNF-α) had increased but only appropriate amount of IL-8 protein had been detected. At high concentration, the secretion of the fibroblasts IL-8 is similar with keratinocytes; The immune response of caused by the imiquimod-induced secretion of cytokines is similar with the DTH response; imiquimod can activate the anti-tumor activity and DTH response of the HPV E7 gene transfected tumor bearing mice; human and animal experiments have shown that imiquimod could increase the 2'5'-oligo-adenylate synthase which can induce the interferon for inducing the antiviral effect; interferons and other cytokines can activate NK cells to kill tumor cells and the virus-infected cells; both in vivo and in vitro tests have showed that the imiquimod can increase activity of NK and stimulate the proliferation and activation of mouse spleen B cells which is the direct effect rather than being mediated by cytokine; imiquimod does not directly enhance T-lymphocyte activity; but in vitro mitogenic response can stimulate T-cells for enhancing the production and proliferation of IL-2. In vivo experiments have demonstrated that it can enhance T cell activity; imiquimod can increase the generation of IL-2 of the HSV infected guinea pig, T cell proliferation and T cell activity to enhance the cell-mediated immune response.
The above information is edited by the lookchem of Dai Xiongfeng.
Mechanism of action
Imiquimod (Aldara) is an imidazoquinolone amine that is an immune responsemodifier.
Its exact mechanism is unknown in the topical treatment of HPV and molluscum
contagiosum but may be related to the immunomodulating effect of the drug. It is also
effective in the treatment of actinic keratoses and superficial basal cell carcinoma.
It induces production of a variety of cytokines and can enhance cell-mediated
cytolytic antiviral activity. It is a rapid and potent inducer of interferon-α,
interleukin?1 α and β, interleukin 6, interleukin 8, tumor necrosis factor-α,
granulocyte-macrophage colony-stimulating factor, and granulocyte colonystimulating
factor. Systemic absorption is minimal. It is generally well tolerated.
Adverse local reactions include erythema, erosion, excoriation, flaking, and edema
of the treatment sites.
Usage and Dosage
It can be used for the treatment of adult external genital and perianal genital warts at 3 times per week. Just before sleep, first apply the product evenly in a thin layer on the surface of the wart and gently massage until the product is completely absorbed. The position of the medication should not be packeted and should be maintained for 6 to 10 hours, and then wash with a neutral soap and clean water for clearing the drugs in the administration site. Wash your hands before and after treatment. 250 mg of cream can be applied to 20cm2 of wart. Avoid excessive application of the drug. Patients should continue the treatment until the wart is completely cleared. Wart can be cleared within 2-4 week at the fastest speed and can be generally cleared within 8 to 12 weeks. The medication should be not more than 16 weeks. After the treatment, patients with mild erythema locally who does not have to be discontinued for drug; if the patients feel general malaise or get local skin reactions (edema, erosion, pain, etc.), the drug should be discontinued. Only when the reaction is alleviated can they continue the medication.
Side effects
1, the majority of patients get no adverse reactions during the treatment. After several times of treatment, the clinical adverse reactions are mostly mild, moderate local skin inflammation. For example, there may be local skin erythema, edema, erosion, ulcers, desquamation, burning, pain, itching and so on.
2, there is occasional transient fever and these symptoms can quickly recover after stopping the drugs. If the reactions are mild, you can continue for the treatment. However, the patients should discontinue the drug on time and should be subject to medical treatment if severe reactions happen.
3, According to the reports of foreign information, possible adverse reactions associated with imiquimod cream include discomfort in the medication site, wart site reactions (burning, pigmentation, inflammation, itching, pain, rash, sensitivity, ulcers, tingling and tenderness).Distal site reactions (bleeding, burning, itching, pain and tenderness), fatigue, fever, flu-like symptoms, headache, diarrhea, myalgia.
Precautions
1. No packets; wash away the drug after 6 to 10 hours of the drug administration. The patients should avoid using this product in partial breakage place. There have been reports of using drugs or laser for treatment of genital warts that lead to the emergence of damaged parts. In that case, we should continue the drugs until the wound get healed.
2. It should not be applied to the treatment of genital warts located at the eyes, mouth, nose, urethra, vagina, cervix and anal mucous membranes.
3. You should avoid sex during the treatment. For patients using condom, the patients should first clean the topical imiquimod because imiquimod can make condom be fragile.
4. For male patients with foreskin, during the treatment, the patients should reveal the foreskin every day and clean the application site. If the foreskin mucosa surface gets erosion, ulceration, edema and difficulty for turning up foreskin, you should immediately stop the treatment.
5. There have been no reports regarding the drug contraindications in pregnant and lactating women, but the patients should use with caution.
Originator
3M Pharmaceuticals (US)
Indications
Imiquimod (Aldara) is a topical immune response modifier
approved for the treatment of anogenital warts
(condylomata acuminata). The exact mechanism of action
is unknown; it has no direct antiviral activity in
vitro. It is thought to work in vivo by inducing the production
of tumor necrosis factor (TNF α ), interferons
(IFN)α and γ , and other cytokines with antiviral activity.
It may also be useful for treatment of other types of
warts, molluscum contagiosum, and certain forms of
skin cancer. Local irritant reactions related to the frequency
of application are common.
Pharmaceutical Applications
An imidazoquinoline used for the treatment of genital and
perianal warts. While the mechanism of action is not precisely
known, it is thought to induce interferon. It has no direct antiviral
activity. The 5% cream applied three times a week for
up to 16 weeks resulted in total wart clearance in 50% of
patients, with a better response in women than in men. Local
reactions are common and include erythema, erosion, excoriation
and edema.
Biological Activity
Immunomodulator that displays antiviral and antitumor activity. Acts as a Toll-like receptor 7 (TLR-7) agonist; stimulates proinflammatory cytokine production and activates NF- κ B.
Biochem/physiol Actions
Imiquimod is a caspase 3 activator, which directly induces procaspase 3 cleavage to active caspase 3. Imiquimod induces apoptosis in vivo in basal cell carcinoma. Its anti-tumor activity is related to the induction of apoptosis. Imiquimod has anti-angiogenic, anti-inflammatory, anti-viral activities. Imiquimod also acts as an immune response modulator inducing the secretion of various cytokines and chemokines.
Veterinary Drugs and Treatments
An immune response modifier, imiquimod may be useful in the treatment of a variety of topical conditions in animals. It is labeled for use
on humans as a treatment for genital or perianal warts, superficial basal cell carcinomas and actinic keratoses of the face and scalp. In dogs
and cats, imiquimod potentially may be of benefit in treating feline herpes virus dermatitis, actinic keratosis, squamous cell carcinoma
and Bowen’s disease, papillomas virus lesions, and localized solar dermatitis or solar carcinoma in situ. In horses, imiquimod has been
anecdotally used with success in treating sarcoids.
Imiquimod stimulates the patient’s own immune system to release a variety of cytokines including interferon-alpha and interleukin-
12. Imiquimod itself does not have in vitro activity against wart viruses, but stimulates monocytes and macrophages to release cytokines
that induce a regression in viral protein production.
References
1) Hemmi?et al.?(2002),?Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway;?Nat. Immunol.?3?196
2) Stanley?et al.?(2002),?Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential;?Clin. Exp. Dermatol.?27?571
3) Schoen?et al.?(2006),?The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion;?J.Invest.Dermatol.?126?1338
4) Kan?et al.?(2012),?Imiquimod Suppresses Propagation of Herpes Simplex Virus 1 by Upregulation of Cystatin A via the Adenosine Receptor A1?Pathway.; J. Virol.?86?10338
5) Urosevic?et al.?(2004),?Imiquimod Treatment Induces Expression of Opioid Growth Factor Receptor; Clin. Cancer Res.?10?4959
6) Zagon?et al.?(2008),?Imiquimod Upregulates the Opioid Growth Factor Receptor to Inhibit Cell Proliferation Independent of Immune Function.; Exp. Biol. Med.(Maywood)?233?968
Check Digit Verification of cas no
The CAS Registry Mumber 99011-02-6 includes 8 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 5 digits, 9,9,0,1 and 1 respectively; the second part has 2 digits, 0 and 2 respectively.
Calculate Digit Verification of CAS Registry Number 99011-02:
(7*9)+(6*9)+(5*0)+(4*1)+(3*1)+(2*0)+(1*2)=126
126 % 10 = 6
So 99011-02-6 is a valid CAS Registry Number.
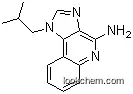
InChI:InChI=1/C14H16N4/c1-9(2)7-18-8-16-12-13(18)10-5-3-4-6-11(10)17-14(12)15/h3-6,8-9H,7H2,1-2H3,(H2,15,17)
99011-02-6Relevant articles and documents
Mild, General, and Regioselective Synthesis of 2-Aminopyridines from Pyridine N -Oxides via N -(2-Pyridyl)pyridinium Salts
Xiong, Hui,Hoye, Adam T.
supporting information, p. 371 - 375 (2022/01/27)
A synthesis of 2-aminopyridines from pyridine N-oxides via their corresponding N-(2-pyridyl)pyridinium salts has been demonstrated and investigated. The reaction sequence features a highly regioselective conversion of the N-oxide into its pyridinium salt
IMIQUIMOD PRODUCTION PROCESS
-
Page/Page column 2-3, (2008/12/07)
The present invention provides a process for preparing highly pure 4-amino-1-isobutyl-1H-imidazo[4,5-c]quinoline (imiquimod). The process preferably includes heating 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline of formula (II) with ammonia in a polar aprotic solvent at relatively moderate pressure to produce imiquimod, and optionally purifying the imiquimod. The process of the present invention can produce highly pure imiquimod in high yield.
Imiquimod Production Process
-
Page/Page column 2; 4, (2008/12/07)
Provided is a process for producing highly pure 4-amino-1-isobutyl-1H-imidazo[4,5-c]quinoline (imiquimod), which includes reacting 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline with a non-gaseous amine precursor. Also provided are methods for isolating highly pure imiquimod. Further provided are intermediates useful in the production of imiquimod, methods for producing such intermediates, and methods for obtaining imiquimod from such intermediates.
Preparation of 1H-imidazo[4,5-c]quinolin-4-amines via 1H-imidazo[4, 5-c]quinolin-4-phtalimide intermediates
-
Page/Page column 6, (2008/06/13)
The present invention provides process for the preparation of 4-amino-1H-imidazo[4,5-c]quinolines comprising the step of reacting a 1H-imidazo[4,5-c]quinolin-4-phthalimide with an amine selected from the group consisting of: alkylamine, aralkylamine, alkyldiamine, and aralkyldiamine.
New process for the manufacture of 1H-imidazo [4,5-c]-quinoline ring systems
-
Page/Page column 17, (2009/01/24)
The present invention relates to a new synthetic route to manufacture 1H-imidazo[4,5-c]-quinoline ring systems and to new corresponding intermediates. Said new intermediates are biaryl aminonitrile compounds of formula (II)
Process for the preparation of imidazo[4,5-c]-quinolin-4-amines
-
Page/Page column 3-4, (2008/06/13)
The present invention is a method for preparing a compound of the formula wherein R is hydrogen, C1 to C6 alkyl, C1 to C6 alkoxy or halo, R1 and R2 are independently hydrogen, C1 to C10 alkyl, C1 to C10 alkoxy, C3 to C10 cycloalkyl, C3 to C10 alkenyl, C5 to C10 cycloalkenyl, C2 to C10 alkynyl, C6 to C20 aryl or substituted C6 to C20 aryl and n is the integer 1 or 2 by reacting the corresponding N-oxide with an aqueous solution of ammonia and a C1 to C6 alkyl, C6 to C20 aryl or substituted C6 to C20 aryl chloroformate thereby forming said compound formula (1).
Process for preparing Imiquimod
-
Page/Page column 4, (2008/06/13)
The present invention provides a process for preparing 4-amino-1-isobutyl-1H-imidazo[4,5-c]quinoline (Imiquimod) of formula (I). The process comprises heating 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline of formula (II) with formamide, and optionally with bubbling of gaseous ammonia to afford Imiquimod of formula (I). According to the present invention, by using this process and novel purification methods, essentially as described herein, highly pure Imiquimod is obtained.
A process for the purification of imiquimod
-
Page/Page column 3, (2008/06/13)
Crystalline forms of imiquimod, a process for the preparation thereof and the use thereof in the purification of imiquimod.
A PROCESS FOR PREPARATION OF 4-AMINO-1-ISOBUTYL-1H-IMIDAZO[4,5-C]-QUINOLINE (IMIQUIMOD)
-
Page/Page column 10-11, (2008/06/13)
A process for preparation of imiquimod comprising oxidation of 1-Isobutyl-1H-imidazo quinoline quinoline (II) afforded 1-Isobutyl-lH- imidazo-[4,5-c]-quinoline-5-N-oxide (III) which is isolated in pure form as its hydrochloride salt (IV) followed by conversion to 4-chloro derivative(V) and conversion to corresponding 4-iodo derivative (VI) which is a new intermediate. This new intermediate is convened to imiquimod (VIII) and purified via its novel maleate salt.
A PROCESS FOR THE PREPARATION OF SUBSTANTIALLY PURE 4-AMINO-1-ISOBUTYL-1H-IMIDAZO[4,5-C]-QUINOLINE (IMIQUIMOD)
-
Page/Page column 7; 13, (2008/06/13)
A process for preparation of Imiquimod comprising oxidation of 1-Isobutyl-1H-imidazo-[4,5-c]-quinoline (II) afforded 1-Isobutyl-1H- imidazo-[4,5-c]-quinoline-5-N-oxide(III) which is isolated in pure form as its hydrochloride salt (IV) followed by conversion to 4-chloro derivative(V) and conversion to corresponding 4-iodo derivative (VI) which is a novel intermediate. This novel intermediate is converted to imiquimod (VIII) and purified via its organic salt. The invention also relates to crystalline polymorphic forms of Imiquimod Maleate, Fumarate and Oxalate.