
Journal of Solution Chemistry p. 775 - 784 (1981)
Update date:2022-08-11
Topics:
 Hoeiland, H.
Hoeiland, H.
 Hald, L.H.
Hald, L.H.
 Kvammen, O.J.
Kvammen, O.J.
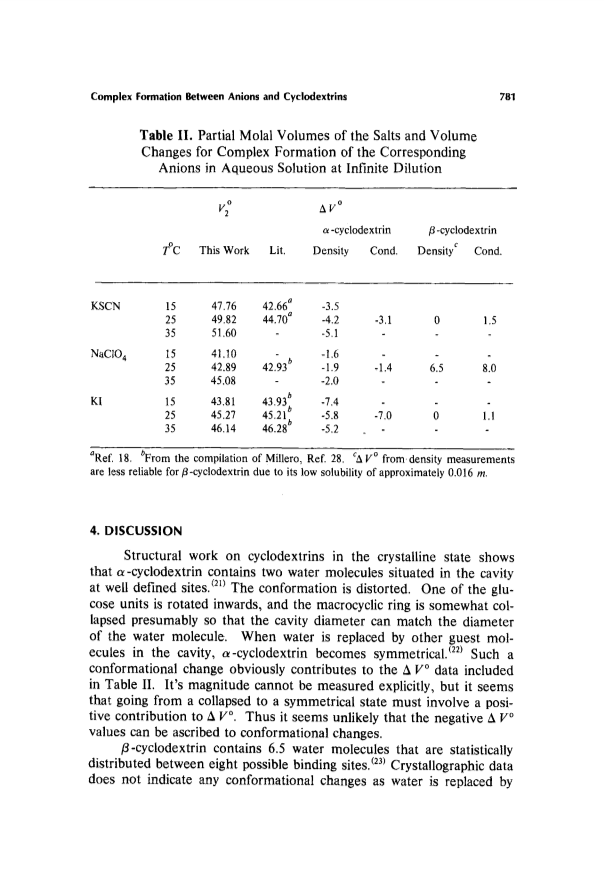
Partial molal volume changes during complex formation between SCN(1-), I(1-), and ClO4(1-) and α- and β-cyclodextrin have been determined by two independent methods of measurements; one based on density measurement and subsequent calculation of apparent molal volumes, the other on differentiating the association constants with respect to pressure.Results from the two methods are in good agreement.Negative volume changes were observed for complex formation between the anions and α-cyclodextrin while zero or slightly positive values were observed for complex formation with β-cyclodextrin.The result is consistent with the idea that the anions do not become dehydrated as they form complexes with cyclodextrins.
View More








GUANGZHOU MEDCAN PHARMATECH LTD
website:http://www.gzmedcan.com
Contact:+86-20-82519649
Address:Building J,Room 101,1 JiangtashanRd,Guang Zhou Science City,Guang Zhou ,China
Shanghai Rich Chemicals Co., Ltd
website:http://www.richchemical.com
Contact:+86-21-20255798
Address:Pudong Shanghai,China
Zhuhai Jiacheng Biological Technology Co., Ltd
Contact:0756-8800233
Address:room 222, Lianhua road , Gongbei ,Zhuhai, Guangdong ,China
website:http://www.simagchem.com
Contact:+86-592-2680277
Address:21/F Hualong Office Building,No.6 Hubin East Road, Xiamen,China
Zibo Xiaoguang Chemical Material Co., Ltd
Contact:15954099116
Address:Boshan Development Zone
Doi:10.1016/j.tetasy.2009.07.048
(2009)Doi:10.1016/j.molcata.2015.05.009
(2015)Doi:10.1021/jo01362a028
(1957)Doi:10.1039/j29710001625
(1971)Doi:10.1021/ja01245a513
(1943)Doi:10.1021/jm00333a045
(1964)