
Journal of Organic Chemistry p. 4928 - 4933 (1990)
Update date:2022-08-11
Topics:
 Asakura, Jun-ichi
Asakura, Jun-ichi
 Robins, Morris J.
Robins, Morris J.
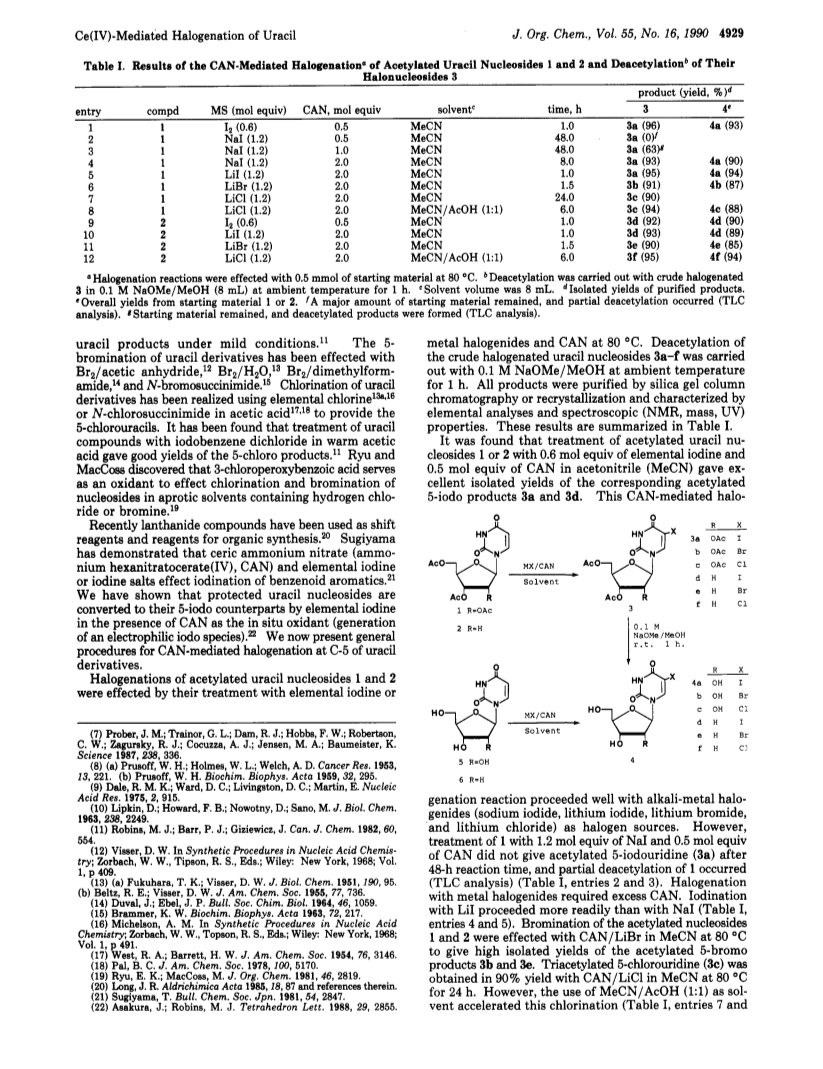
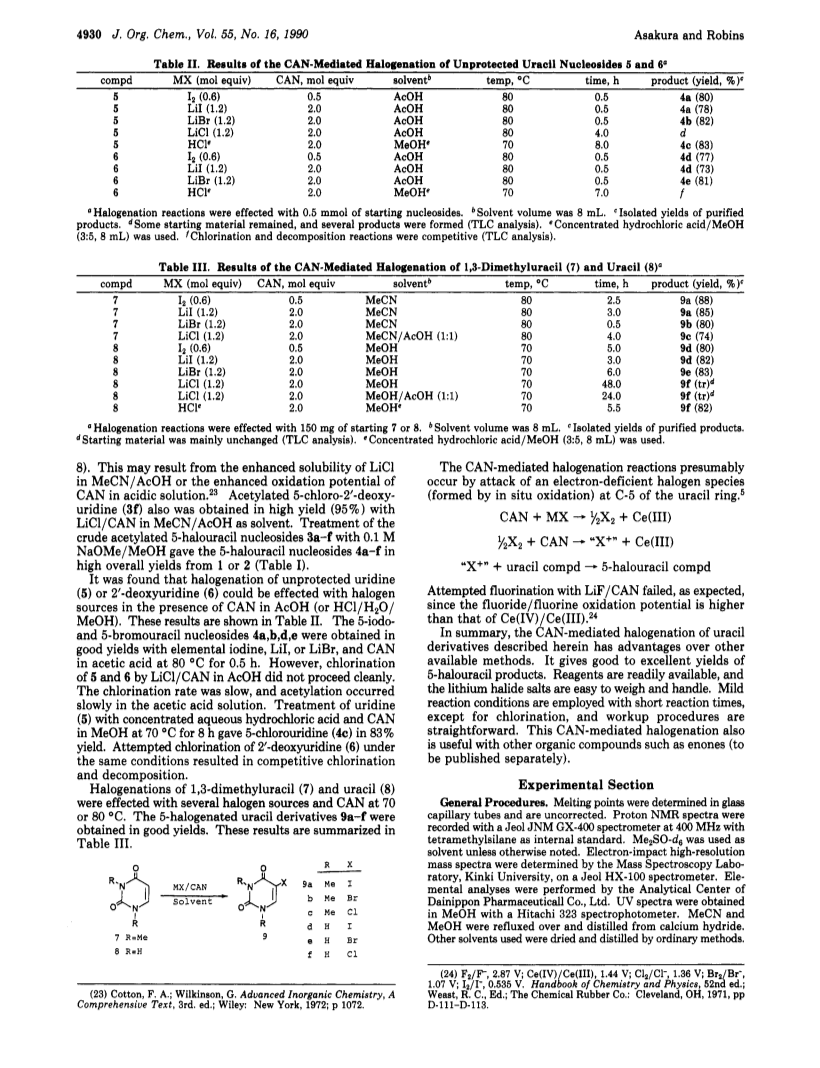
Treatment of protected uracil nucleosides 1 or 2 with elemental iodine or metal halogenides and ceric ammonium nitrate (CAN) at 80 deg C gave the corresponding protected 5-halouracil nucleosides 3a-f in excellent yields.Treatment of the resulting crude 3a-f with 0.1 M NaOMe/MeOH at ambient temperature gave the corresponding 5-halouridines 4a-f in high overall yields from 1 or 2.Further, 5-halouraciles 9a-f were prepared in good yields by treatment of 1,3-dimethyluracil (7) or uracil (8) with elemental iodine, metal halogenides, or hydrochloric acid and CAN.Halouridines 4a-e also were obtained in good yields by treatment of unprotected uracil nucleosides 5 or 6 with halogen sources as above and CAN.
View More



NINGBO PANGS CHEM INT’L CO.,LTD.
Contact:+86-574-27666845
Address:FLOOR 21,BUILDING NO.11,XIN TIAN DI,NO.689 SHI JI ROAD,NINGBO CHINA
JiYi Chemical (Beijing) Co., Ltd.
Contact:+86-10-89385733
Address:Shilou Town of Fangshan District, Beijing
Suzhou Time-chem Technologies Co., Ltd.
Contact:0512-63983931/68086856
Address:No. 1326 of Binhe Road, New District, Suzhou, Jiangsu, P. R. China
Contact:+91 9963263336
Address:Plot#146A, IDA Mallapur, Hyderabad - 500072
Shandong Hongxiang Zinc Co., Ltd
Contact:086-0311-66187879
Address:DaWang developing zone
Doi:10.1021/jo961357h
(1996)Doi:10.1016/j.tetlet.2009.09.171
(2009)Doi:10.1021/np000506v
(2001)Doi:10.1039/b109249a
(2001)Doi:10.1016/j.tet.2013.02.093
(2013)Doi:10.1021/jo00147a023
(1982)