
Journal of the Brazilian Chemical Society p. 1509 - 1516 (2010)
Update date:2022-08-30
Topics:
 Vieira, Gizelle A. B.
Vieira, Gizelle A. B.
 De Freitas Araujo, Daniel M.
De Freitas Araujo, Daniel M.
 Lemos, Telma L. G.
Lemos, Telma L. G.
 De Mattos, Marcos Carlos
De Mattos, Marcos Carlos
 Da Conceic?a?o F. De Oliveira, Maria
Da Conceic?a?o F. De Oliveira, Maria
 Melo, Va?nia M. M.
Melo, Va?nia M. M.
 De Gonzalo, Gonzalo
De Gonzalo, Gonzalo
 Gotor-Ferna?ndez, Vicente
Gotor-Ferna?ndez, Vicente
 Gotor, Vicente
Gotor, Vicente
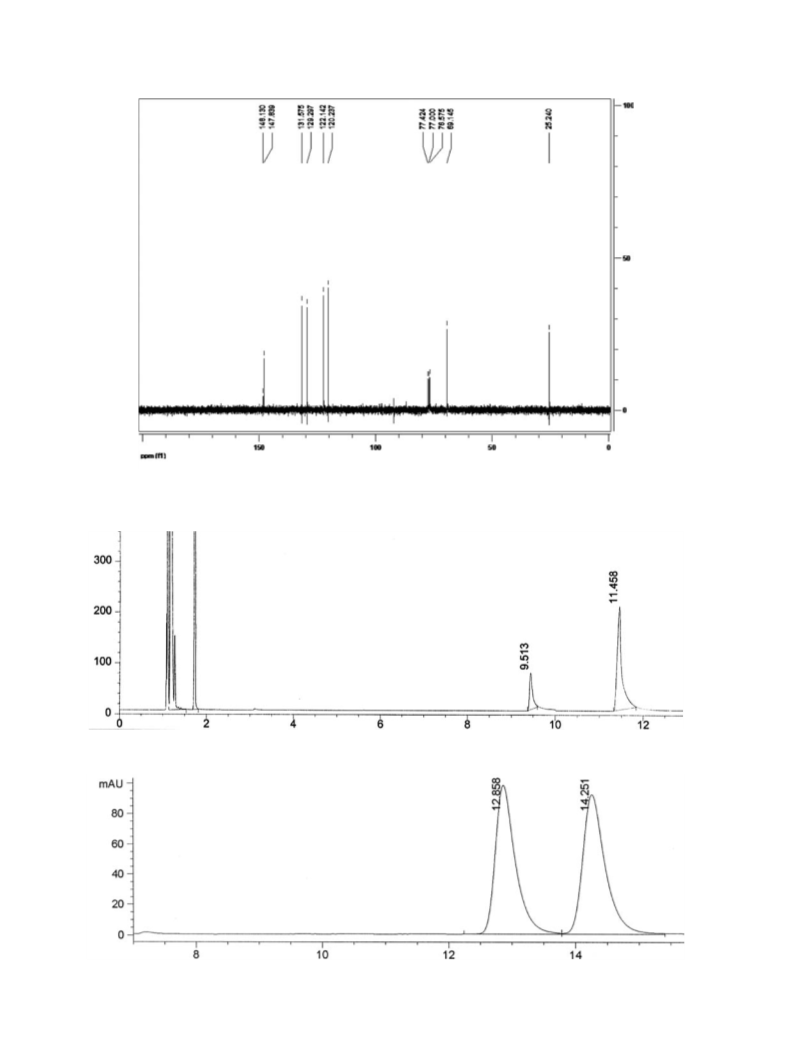
The reactivity and stereoselectivity showed by a new strain of Candida tropicalis in the reduction of prochiral ketones have been compared with the ones previously attained in our laboratory using microorganisms from the Brazilian biodiversity. In this manner, Candida tropicalis has demonstrated its versatility as stereoselective agent in the bioreduction of a series of aromatic ketones. These prochiral compounds were converted into their corresponding optically alcohols with moderate to excellent stereopreference depending on the substrate structure. Among ketones tested, nitroacetophenones were enzymatically reduced to enantiopure (S)-alcohol with complete conversion.
View More
























ZHEJIANG CHEMICAL INDUSTRY INSTITUTE TECHNOLOGY CO.,LTD(expird)
Contact:86-575-82730298
Address:shangyu
jiangsu senxuan pharmaceutical and chemical co.,ltd
Contact:86-523-87982810
Address:hongqiao industrial zone,taixing,jiangsu china
Shanghai Hongbang Medical Technology CO.,. Ltd
Contact:13671516988 /18917636693
Address:Room1, No67 Building, Yongde Road369, Wujing Town, Minhang Districy, Shanghai CIty, China.
Chongqing Rong&Quan Pharmaceutical Technology Co. , Ltd.
Contact:86-023-65268721
Address:No. 7, Manshanhong Village, Pingdingshan, Shapingba District, Chongqing Province, China
Lianyungang Yunbo Chemical Co.,Ltd.
Contact:518-81066110
Address:B907,Dongsheng"Mingdu Square,21-2 East Chaoyang Road,Xinpu,(sale department)
Doi:10.1021/jacs.0c05804
(2020)Doi:10.1016/0022-2852(68)90023-4
(1968)Doi:10.1002/pola.24692
(2011)Doi:10.1126/science.133.3470.2067
(1961)Doi:10.1080/15421406.2014.905087
(2015)Doi:10.1007/BF00629774
(1991)