
Research on Chemical Intermediates p. 1143 - 1152 (2013)
Update date:2022-08-17
Topics:
 Liu, Jin-Qiang
Liu, Jin-Qiang
 Chen, Xin-Zhi
Chen, Xin-Zhi
 Ji, Baoming
Ji, Baoming
 Zhao, Bang-Tun
Zhao, Bang-Tun
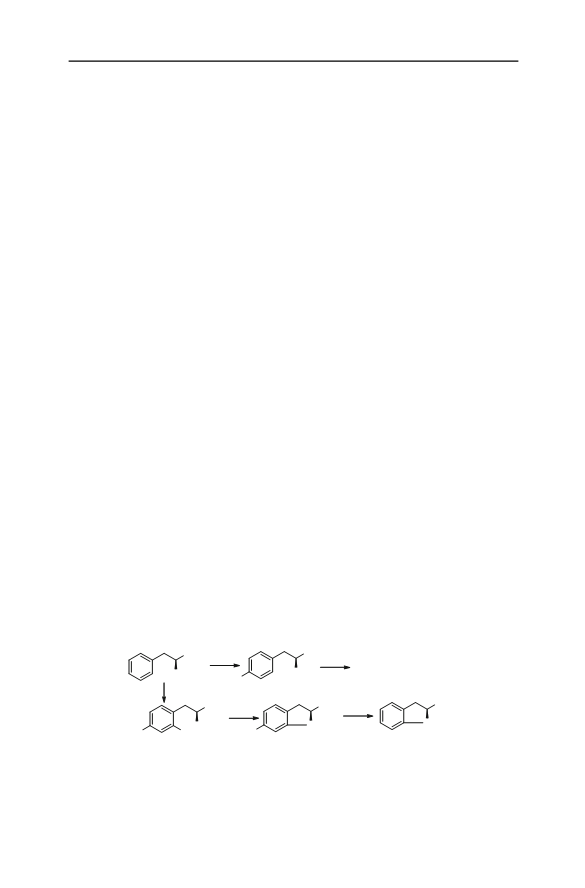
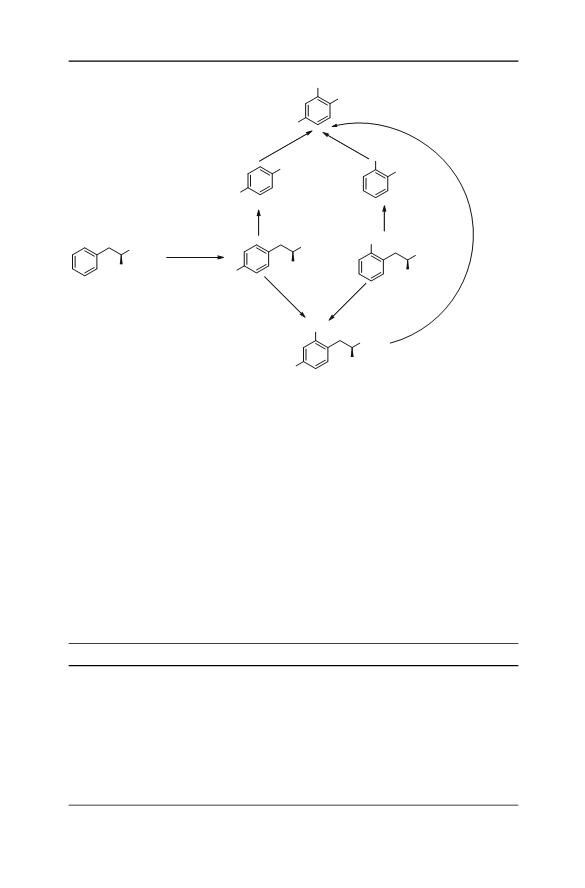
(S)-Indoline-2-carboxylic acid was synthesized by use of a nitro amination approach with l-phenylalanine as chiral pool. The first step of the synthesis was nitration of l-phenylalanine, with urea nitrate (UN)/H2SO 4 as nitrating reagent, to give 2,4-dinitro-l-phenylalanine in 75.7 % yield in one-pot synthesis and 69.1 % yield by step-wise nitration. Intramolecular nitro amination of 2,4-dinitro-l-phenylalanine gave (S)-6-nitro-indoline-2-carboxylic acid in 65.7 % yield and more than 99.5 % enantiomeric excess (ee). The title compound, (S)-indoline-2-carboxylic acid, was obtained in 85.9 % yield and high ee by one-pot transformation of (S)-6-nitroindoline-2-carboxylic acid. The total synthesis consisted of three operations and gave the title compound in 42 % yield and more than 99.5 % ee.
View More






ZHEJIANG PAULA INDUSTRY CO.,LTD
website:http://www.haiqiangchem.com
Contact:86-571-86960380
Address:6th floor, building 2, dongfangmao business center, Xiacheng District, Hangzhou City, Zhejiang Province
chengdu firsterchem Pharmaceutical Co., Ltd.
Contact:028-66825849
Address:chengdu
JiYi Chemical (Beijing) Co., Ltd.
Contact:+86-10-89385733
Address:Shilou Town of Fangshan District, Beijing
Yantai Derun Liquid Crystal Materials Co. Ltd.
website:http://www.ytderun.com
Contact:86-535-6300169
Address:ROOM 90, XIANGFU STREET, FUSHAN NEW-HIGH-TECH IDUSTRY ZONE, YANTAI
Quzhou Hopemax Chemical Technology Co.Ltd
Contact:86-570-3036007
Address:Room 801,Building 2,Wealth Center,No.51,Xujiang Road,Quzhou,China.
Doi:10.1016/S0013-4686(03)00513-9
(2003)Doi:10.1016/S0040-4039(99)02129-2
(2000)Doi:10.1016/S0040-4039(00)77478-8
(1993)Doi:10.1002/chem.201302085
(2013)Doi:10.1039/b207885f
(2002)Doi:10.1021/ja01628a097
(1955)