
Chemistry of Heterocyclic Compounds p. 322 - 325 (1985)
Update date:2022-08-11
Topics:
 Wroczynski, P.
Wroczynski, P.
 Kujawa, A.
Kujawa, A.
 Skul'ski, L.
Skul'ski, L.
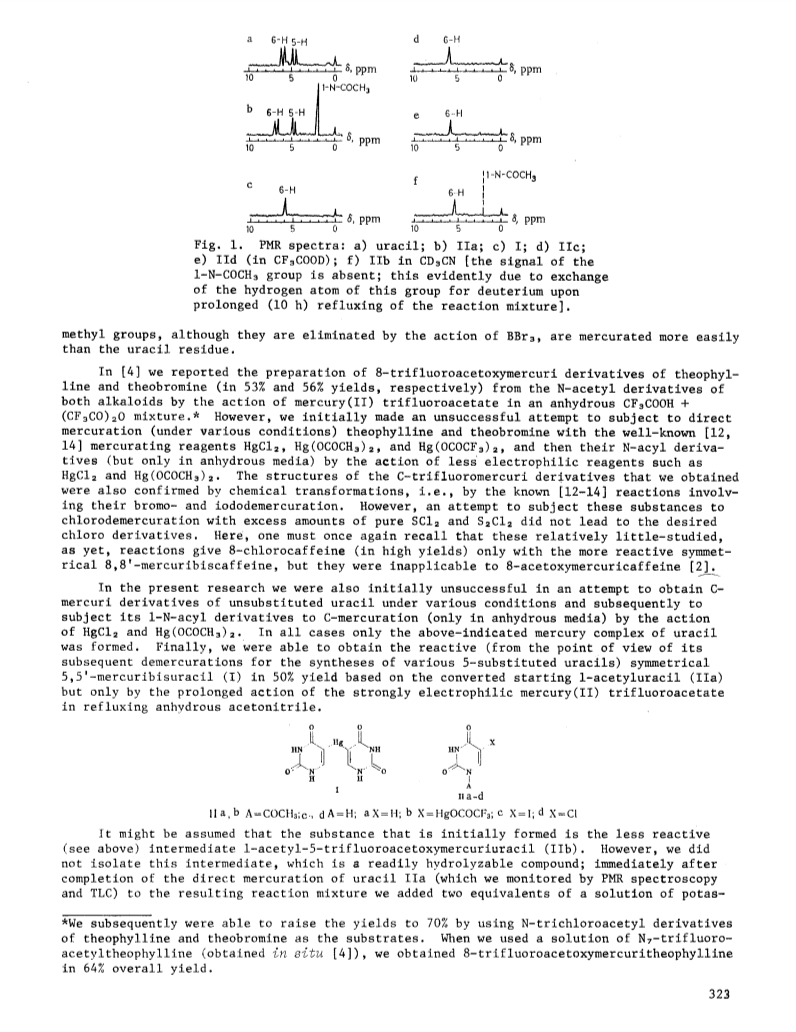
The direct C mercuration in the 5 position of 1-acetyluracil by means of mercury(II) trifluoroacetate in anhydrous acetonitrile is described.The intermediately formed 1-acetyl-5-trifluoroacetoxymercuriuracil, without isolation, was subjected to symmetrization by the action of potassium iodide.The acetyl groups were then readily split out by the action of water.The resulting 5,5'-mercuribisuracil (50percent yield) forms 5-iodo- or 5-chlorouracil in 93percent or 72percent yields, respectively, under the influence of an aqueous KI3 solution or excess pure liquid S2Cl2.
View More


SHUNYUANSHENG BIO-PHARMTECH CO., LTD
website:https://www.whsysbio.com
Contact:--
Address:Building 13, Liandong U Valley-Wuhan Economic Innovation Valley, No. 259, Xingsan Road, Shamao Street, Hannan District, Wuhan City, Hubei Province
website:http://www.u-chemo.com
Contact:+86-21-61558312
Address:Dong Fang Road,
Hangzhou Maytime Bio-Tech Co.,Ltd.
website:http://www.maytime.com.cn
Contact:+86-571-88925295 88920965
Address:NO.2-1701 Ganghui Central Ningwei Street, Xiaoshan Hangzhou Zhejiang China
Shandong Jincheng Zhonghua Bio-pharmaceutical Co.,Ltd
Contact:+86-533-5415882
Address:Zichuan Economic Development Zone,Zibo City,Shandong Province,China
Zhengzhou Xinlian Chemical Tech Co. ,Ltd
Contact:0371-65771781 021-52042910
Address:H Part, Building I, No. 700 Gonglu Road, Pudong New Area, Shanghai
Doi:10.1016/j.jpcs.2004.04.007
(2004)Doi:10.1021/jf1035433
(2011)Doi:10.1039/CT9130300361
(1913)Doi:10.1002/cphc.201601053
(2017)Doi:10.1021/acscatal.6b03434
(2017)Doi:10.1039/c5ra16614d
(2015)