
Bulletin of the Academy of Sciences of the USSR Division of Chemical Science p. 1677 - 1682 (1989)
Update date:2022-08-12
Topics:
 Volod'kin, A. A.
Volod'kin, A. A.
 Zaitsev, A. S.
Zaitsev, A. S.
 Rubailo, V. L.
Rubailo, V. L.
 Belyakov, V. A.
Belyakov, V. A.
 Zaikov, G. E.
Zaikov, G. E.
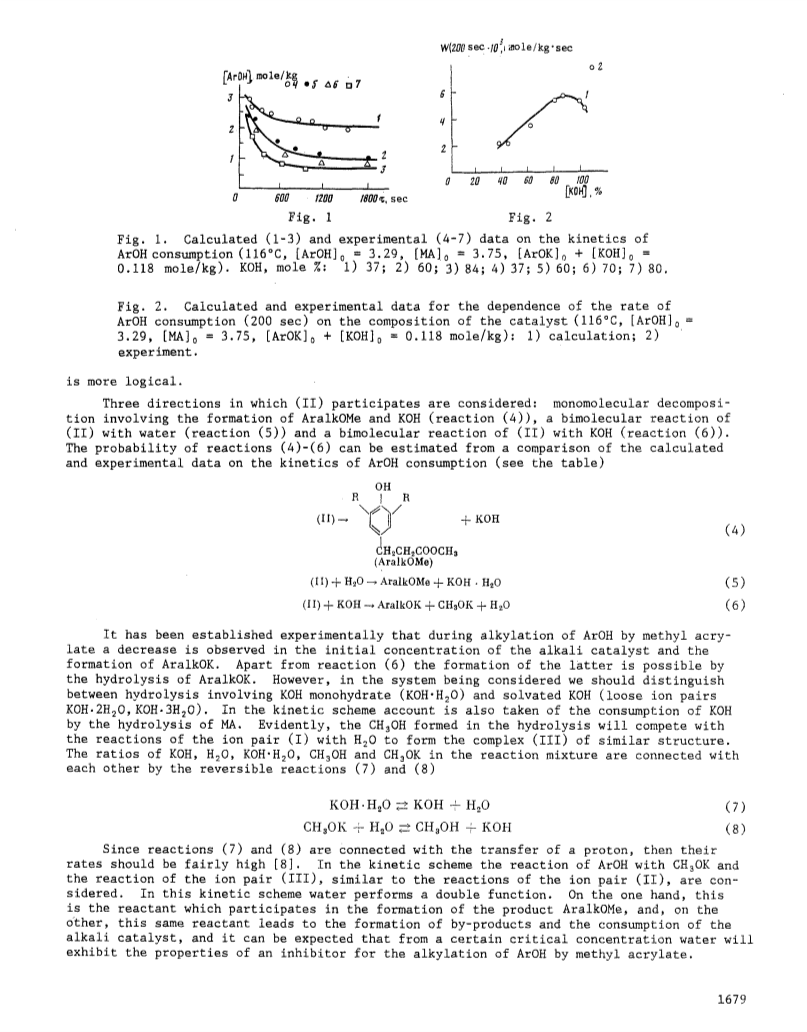
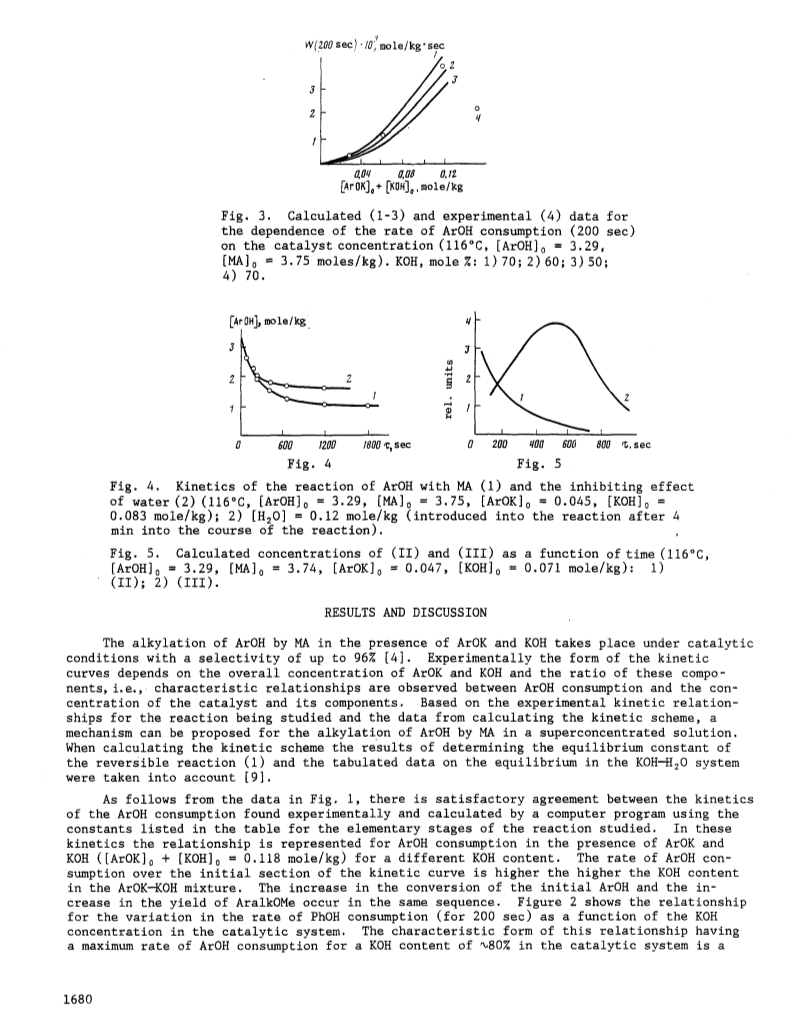
A mechanism is proposed for the reaction of 2,6-di-tert-butylphenol with methyl acrylate in the presence of potassium 2,6-di-tert-butylphenoxide and KOH and, based on a computer calculation of the kinetic scheme, the constants have been found for the elementary stages of the reaction, sufficient to interpret the experimental data.The alkylation of 2,6-di-tert-butylphenol by methyl acrylate takes place by an inhibited catalytic chain mechanism involving the participation of ion pairs in the reaction.
View More

Contact:+86 180 6092 1829
Address:102-31, No. 1 Bldg, No. 1888, HongXiang West Road, XiangAn
Shijiazhuang Sdyano Fine Chemical Co., Ltd
Contact:+86-311-89830448
Address:NO.48 Ta Nan Road,Yuhua District,Shijiazhuang,Hebei,China
Contact:+49-4101-3053-0
Address:Waldhofstrasse 14 ,25474 Ellerbek Germany
website:http://www.maisonchem.com.cn
Contact:0086-311-83833777
Address:Leitou industrial district, xinji, shijiazhuang city, hebei province,
zhuzhou zhongle chemical co. ltd.
Contact:+86-0731 28228409
Address:Zhuzhou, Hunan, China
Doi:10.1016/j.electacta.2005.03.049
(2005)Doi:10.1016/j.tetlet.2017.02.077
(2017)Doi:10.1039/c7cc05297a
(2017)Doi:10.1039/jr9500002100
(1950)Doi:10.1039/b101434j
(2001)Doi:10.1021/ic101528d
(2010)