
Journal of the American Chemical Society p. 5514 - 5519 (1987)
Update date:2022-08-17
Topics:
 Chari
Chari
 Whitman
Whitman
 Kozarich
Kozarich
 et al.
et al.
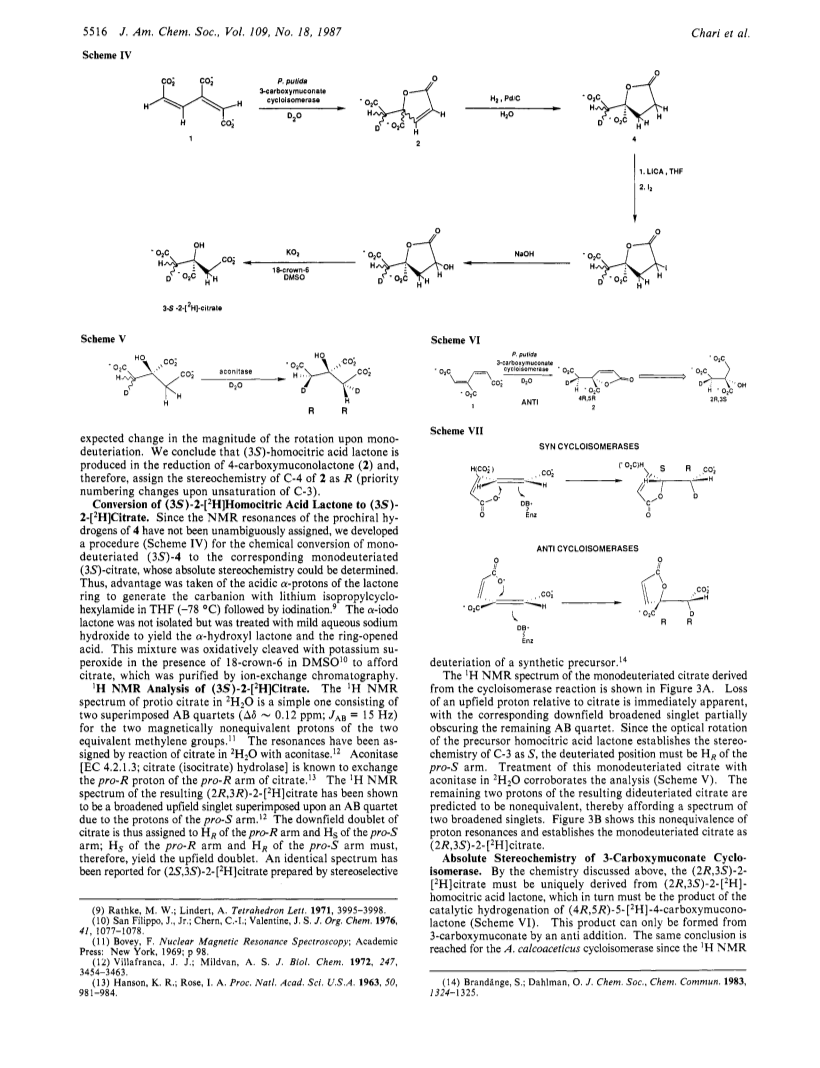
The absolute stereochemical course of the 3-carboxymuconate cycloisomerases [EC 5.5.1.2; 2-carboxy-5-oxo-2,5-dihydrofuran-2-acetate lyase (decyclizing)] from Pseudomonas putida and Acinetobacter calcoaceticus has been determined by chemical and 1H NMR methods. The product of the enzyme-catalyzed reaction in 2H2O was detected by NMR and trapped by catalytic hydrogenation to afford 5-[2H]homocitrate lactone. Subsequent chemical degradation of the monodeuteriated homocitrate lactone gave (2r,3S)-2-[2H]citrate as determined by 1H NMR analysis. The product of the cycloisomerase reaction was established as (4R,5R)-5-[2H]-4-carboxymuconate, indicating that the lactonization proceeded by an anti addition - the mechanistic and stereochemical antipode of the previously studied muconate cycloisomerase from P. putida and 3-carboxymuconate cycloisomerase from Neurospora crassa. The anti addition probably represents the lower energy pathway for the reaction and suggests that the evolutionary relationship between the two classes of cycloisomerases is more remote than previously believed.
View More




Nanjing Samwon International Limited
Contact:+86-25-84873444
Address:1108, BLDG B, New Century Plaza, No 1, South Taiping Rd.,
hangzhou verychem science and technology co.ltd
website:http://www.verypharm.com
Contact:+86-571-88162785; 88162786
Address:F1502, 753 Shenhua road, Hangzhou, China
Contact:+1-973-357-0577
Address:10 Taft Rd.
Contact:+86-18321548194
Address:3Rd.Floor, No.780 Cai Lun Road, Pudong New Area, Shanghai, China
lianyungang jinkang pharmaceutical technology co., ltd.
Contact:008651885445517
Address:Jinshan industrial park, Ganyu county, Lianyungang, Jiangsu Province, 222115, China
Doi:10.1039/a903876k
(1999)Doi:10.1016/S0040-4039(01)87617-6
(1973)Doi:10.1016/j.tetlet.2016.05.062
(2016)Doi:10.1016/j.ssc.2005.03.003
(2005)Doi:10.1016/j.ejmech.2016.01.036
(2016)Doi:10.1039/c5gc00580a
(2015)