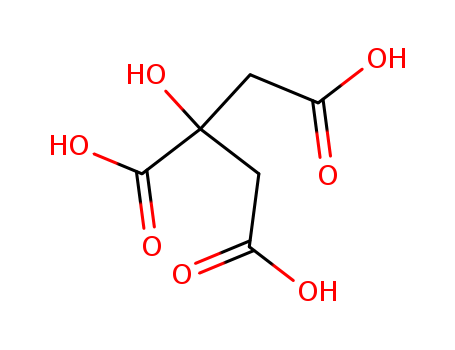
- Chemical Name:Citric Acid
- CAS No.:77-92-9
- Deprecated CAS:12262-73-6,43136-35-2,245654-34-6,623158-96-3,856568-15-5,878903-72-1,890704-54-8,896506-46-0,906507-37-7,1192555-95-5,136108-93-5,2023788-69-2,1192555-95-5,136108-93-5,2023788-69-2,245654-34-6,43136-35-2,623158-96-3,856568-15-5,878903-72-1,890704-54-8,896506-46-0,906507-37-7
- Molecular Formula:C6H8O7
- Molecular Weight:193.117
- Hs Code.:2918.14 Oral rat LD50: 3000 mg/kg
- European Community (EC) Number:201-069-1,920-306-6,921-454-4,680-681-4
- ICSC Number:0855
- NSC Number:759606,626579,30279
- UNII:XF417D3PSL
- DSSTox Substance ID:DTXSID3020332
- Nikkaji Number:J2.824J
- Wikipedia:Citric acid
- Wikidata:Q159683
- NCI Thesaurus Code:C80088
- RXCUI:221946
- Pharos Ligand ID:S2C9CQ2VZQ1D
- Metabolomics Workbench ID:37071
- ChEMBL ID:CHEMBL1261
- Mol file:77-92-9.mol
Synonyms:Anhydrous Citric Acid;Citrate;Citric Acid;Citric Acid Monohydrate;Citric Acid, Anhydrous;Uralyt U


 Xi,
Xi, C
C


