- Phenethyl 2-methylbutyrate
- Phenethyl acetate
- Phenethyl alcohol
- Phenethyl benzoate
- Phenethyl butyrate
- Phenethyl caffeate
- Phenethyl chloracetate
- Phenethyl chloride
- Phenethyl cinnamate
- PHENETHYL ISOBUTYRATE
- 60129-59-17H-Pyrrolo[2,3-d]pyrimidin-4-amine,7-(2-deoxy-b-D-erythro-pentofuranosyl)-
- 60129-60-4Kaur-16-en-18-oic acid,13-(b-D-glucopyranosyloxy)-, (4a)-
- 60129-64-8Kaur-16-en-18-oic acid,11,15-dihydroxy-,â-D-glucopyranosyl ester,(4R,11â,15â)-
- 6012-97-1Thiophene,2,3,4,5-tetrachloro-
- 60132-35-6Pterodondiol
- 60133-17-7D-Glutamine,N-(4-nitrophenyl)-
- 60133-18-81alpha,25-Dihydroxyvitamin D2
- 60134-26-1D-erythro-Pentofuranoside,methyl 2-deoxy-
- 601-34-3Cholest-5-en-3-ol (3b)-, 3-hexadecanoate
- 60137-06-619-Norlanosta-1,5-diene-3,11,22-trione, 16,24-epoxy-2,25-dihydroxy-9-methyl-, (9beta,10alpha,16alpha,24S)-
Hot Products
- 117704-25-3Avermectin A1a,25-cyclohexyl-5-O-demethyl-25-de(1-methylpropyl)-
- 67-03-8Thiazolium,3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methyl-chloride (1:1), hydrochloride (1:1)
- 77-73-64,7-Methano-1H-indene,3a,4,7,7a-tetrahydro-
- 100-06-14'-Methoxyacetophenone
- 766-36-92H-Pyrrol-2-one,3-ethyl-1,5-dihydro-4-methyl-
- 554-13-2Lithium carbonate
- 75-89-82,2,2-Trifluoroethanol
- 485-35-8Cytisine
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
Phenethyl alcohol |
EINECS | 200-456-2 |
| CAS No. | 60-12-8 | Density | 1.02 g/cm3 |
| PSA | 20.23000 | LogP | 1.22140 |
| Solubility | 20 g/L (20 °C) in water | Melting Point |
-27 °C(lit.) |
| Formula | C8H10O | Boiling Point | 218.199 °C at 760 mmHg |
| Molecular Weight | 122.167 | Flash Point | 98.4 °C |
| Transport Information | UN 2810 6.1/PG 3 | Appearance | colorless liquid |
| Safety | 26-28-36/37-36/37/39 | Risk Codes | 21/22-36/38 |
| Molecular Structure |
|
Hazard Symbols |
 Xn Xn
|
| Synonyms |
Phenethylalcohol (8CI);(2-Hydroxyethyl)benzene;2-Phenethanol;2-Phenethyl alcohol;2-Phenyl-1-ethanol;Benzyl carbinol;Ethanol, 2-phenyl-;NSC 406252;PEA;Phenethanol;b-(Hydroxyethyl)benzene;b-PEA;b-Phenethanol;b-Phenethyl alcohol;b-Phenylethanol;b-Phenylethyl alcohol; |
Article Data | 1023 |
Phenethyl alcohol Synthetic route

| Conditions | Yield |
|---|---|
| With morpholine-borane; boron trifluoride diethyl etherate In diethyl ether for 2h; Product distribution; Ambient temperature; | 100% |
| With ammonium formate; palladium on activated charcoal In methanol for 2h; Heating; | 100% |
| With hydrogen In methanol at 25℃; under 750.075 Torr; Reagent/catalyst; Flow reactor; regioselective reaction; | 100% |
- 14629-58-4
trimethyl(phenethyloxy)silane

- 60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With Dowex 1-X8 In ethanol for 8h; Ambient temperature; | 100% |
| With bismuth(lll) trifluoromethanesulfonate In methanol at 20℃; for 0.0166667h; | 98% |
| With methanol; 1,3-disulfonic acid imidazolium hydrogen sulfate at 20℃; for 0.0833333h; Green chemistry; | 98% |
- 78926-09-7
1-tert-butyldimethylsilyloxy-2-phenylethane

- 60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With iron(III) chloride In methanol at 23℃; for 3.5h; | 100% |
| With water; scandium tris(trifluoromethanesulfonate) In acetonitrile for 1h; Ambient temperature; | 98% |
| sulfonic acid functionalized nanoporous silica In methanol at 35℃; for 1.5h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; hydrogen In ethanol at 40℃; under 7600.51 Torr; for 19h; Solvent; Autoclave; Inert atmosphere; | 100% |
| With magnesium sulfate In tetrahydrofuran; dichloromethane | 92% |
| With magnesium sulfate In tetrahydrofuran; dichloromethane | 92% |

| Conditions | Yield |
|---|---|
| With phosphate buffer; Phenyl acetate In diethyl ether for 2.75h; Ambient temperature; pig liver acetone powder; | A 18% B 100% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; silica gel In hexane for 3h; Heating; | 100% |
| With methanol; sodium tetrahydroborate In diethyl ether at 20℃; for 38h; Reduction; | 96% |
| With sodium tetrahydroborate In diethylene glycol dimethyl ether at 104℃; | 95% |
- 14629-62-0
1-(triethylsiloxy)-2-phenylethane

- 60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With iron(III) chloride In methanol at 23℃; for 0.0833333h; | 100% |
| With methanol; trimethylsilyl bromide at 20℃; for 0.166667h; chemoselective reaction; | 98% |
| With Selectfluor In acetonitrile at 150℃; for 0.05h; Microwave irradiation; | 82% |
| With iron(III) p-toluenesulfonate hexahydrate In methanol at 20℃; for 0.333333h; | 80% |
| With hydrogenchloride In methanol at 20℃; for 16h; |
- 1093198-50-5
C24H20O3

- 60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With (triphenylphosphine)gold(I) chloride; silver trifluoromethanesulfonate In ethanol; benzene at 20℃; for 0.3h; | 100% |
- 501014-38-6
allyl 2-phenylethyl carbonate

- 60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| [RuCp(η3-C3H5)(QA)]PF6, QA=quinaldic acid In methanol at 30℃; for 0.5h; | 99% |
| With Fe3O4@SiO2-[(4-(5-O3Si-pentylcarbamoyl)-2-pyridinecarboxylato)CpRu(η3-C3H5)]PF6 In methanol at 30℃; for 2h; Inert atmosphere; chemoselective reaction; | 99.9% |
| [RuCp(η3-C3H5)(QA)]PF6, QA=quinaldic acid In methanol at 30℃; for 0.5h; Product distribution; Further Variations:; Solvents; | 99 % Spectr. |
- 100-41-4
ethylbenzene

A
- 123-07-9
4-Ethylphenol

B
- 98-85-1, 13323-81-4
1-Phenylethanol

C
- 60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With rabbit liver microsomal cytochrome P-450 In water at 25℃; for 12h; | A 0.13% B 99.8% C 0.08% |
Phenethyl alcohol Consensus Reports
Reported in EPA TSCA Inventory.
Phenethyl alcohol Specification
1. Introduction of Phenethyl alcohol
Phenethyl alcohol is colourless liqui, it is an alcohol with a pleasant floral odor. The IUPAC Name of it is 2-phenylethanol.
2. Properties of Phenethyl alcohol
Index of Refraction: 1.535
Molar Refractivity: 37.33 cm3
Molar Volume: 119.7 cm3
Polarizability: 14.8×10-24cm3
Surface Tension: 39.6 dyne/cm
Density: 1.02 g/cm3
Flash Point: 98.4 °C
Enthalpy of Vaporization: 48.05 kJ/mol
Boiling Point: 218.2 °C at 760 mmHg
Vapour Pressure: 0.0741 mmHg at 25°C
Melting Point: −27 °C(lit.)
Water Solubility: 20 g/L (20 ºC)
Stability: Stable. Substances to be avoided include strong acids and strong oxidizing agents. Combustible.
Physical Appearance: colourless liquid
Product Categories: Miscellaneous
Synonyms of Benzeneethanol (CAS NO.60-12-8): 1-Phenyl-2-ethanol ; 2-Hydroxyethylbenzene ; 2-Phenethyl alcohol ; 2-Phenylethanol ; Ethanol, 2-phenyl- ; Methanol, benzyl- ; beta-Fenethylalkohol ; beta-Hydroxyethylbenzene ; beta-Phenylethanol
3. Structure Descriptors of Phenethyl alcohol
InChI=1S/C8H10O/c9-7-6-8-4-2-1-3-5-8/h1-5,9H,6-7H2
4. Toxicity of Phenethyl alcohol
| 1. | eye-rbt 12 g/10M MLD | ARZNAD Arzneimittel-Forschung. Drug Research. 9 (1959),349. | ||
| 2. | skn-gpg 100 mg MLD | FCTXAV Food and Cosmetics Toxicology. 13 (1975),903. | ||
| 3. | mmo-smc 1000 ppm | GENRA8 Genetical Research. 13 (1969),107. | ||
| 4. | uns-mus:ast 8360 µmol/L | BCPCA6 Biochemical Pharmacology. 22 (1973),2511. | ||
| 5. | orl-rat LD50:1790 mg/kg | FCTXAV Food and Cosmetics Toxicology. 2 (1964),327. | ||
| 6. | scu-mus LDLo:1640 mg/kg | JPETAB Journal of Pharmacology and Experimental Therapeutics. 14 (1920),211. | ||
| 7. | skn-rbt LD50:790 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 28 (1974),313. | ||
| 8. | orl-rbt LDLo:2 g/kg | JEENAI Journal of Economic Entomology. 48 (1955),139. |
5. Safety Information of Phenethyl alcohol
Moderately toxic by ingestion and skin contact. A skin and eye irritant. Experimental teratogenic effects. Other experimental reproductive effects. Causes severe central nervous system injury to experimental animals. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes:
 Xn
Xn Risk Statements: 21/22-36/38
21/22: Harmful in contact with skin and if swallowed
36/38: Irritating to eyes and skin
Safety Statements: 26-28-36/37-36/37/39
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36/37: Wear suitable protective clothing and gloves
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
RIDADR: UN 2810 6.1/PG 3
WGK Germany: 1
HazardClass: 6.1
PackingGroup: III
6. Preparation of Phenethyl alcohol
Phenethyl alcohol can be made by a number of procedures; the Grignard reaction is used generally:
C6H5Br + Mg → C6H5MgBr
C6H5MgBr + CH2CH2O → C6H5CH2CH2OMgBr
C6H5CH2CH2OMgBr + H+ → C6H5CH2CH2OH
However, the Friedel-Crafts reaction is also employed to manufacture this particular chemical.
C6H6 + CH2CH2O (+ AlCl3) → C6H5CH2CH2OH (+AlCl3)
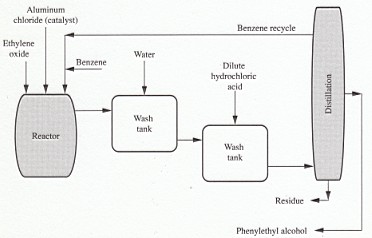
FIGURE 1 Manufacture of phenylethyl alcohol.
7. Use of Phenethyl alcohol
Phenethyl alcohol is much used in perfume formulation. It is used as an additive in cigarettes. It is also used as a preservative in soaps due to its stability in basic conditions. In biology it is of interest due to its antimicrobial properties.

