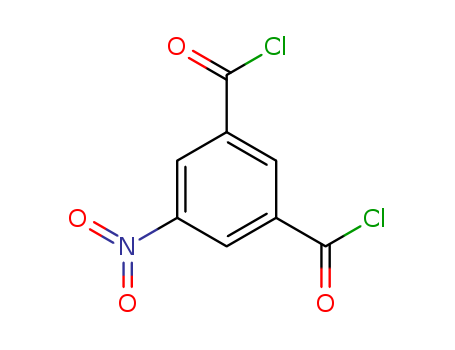
- Chemical Name:5-Nitroisophthaloyl chloride
- CAS No.:13438-30-7
- Molecular Formula:C8H3Cl2NO4
- Molecular Weight:248.022
- Hs Code.:
- European Community (EC) Number:236-575-1
- DSSTox Substance ID:DTXSID90158680
- Nikkaji Number:J28.753I
- Wikidata:Q83026998
- Mol file:13438-30-7.mol
Synonyms:5-Nitroisophthaloyl chloride;13438-30-7;5-Nitroisophthaloyl dichloride;5-nitrobenzene-1,3-dicarbonyl chloride;1,3-Benzenedicarbonyldichloride, 5-nitro-;EINECS 236-575-1;C8H3Cl2NO4;5-Nitroisopthaloyl chloride;SCHEMBL728757;C8-H3-Cl2-N-O4;5-Nitroisophthaloyl dichloride #;DTXSID90158680;5-nitro-isophthalic acid dichloride;5-nitro-1,3-benzenedicarbonylchloride;5-nitro-1,3-benzenedicarbonyl chloride;5-nitro-1,3-benzenedicarbonyl dichloride;AS-76315;1,3-Benzenedicarbonyl dichloride, 5-nitro-;D85792;1,3-BENZENEDICARBONYL DICHLORIDE,5-NITRO;A806943