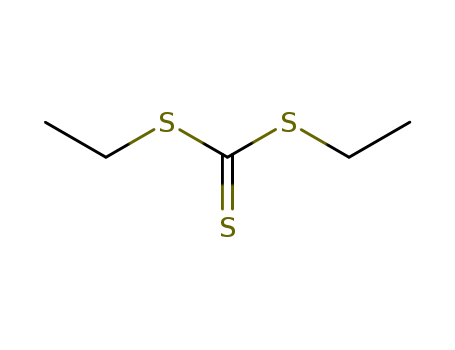
- Chemical Name:Diethyl trithiocarbonate
- CAS No.:2314-49-0
- Molecular Formula:C5H10 S3
- Molecular Weight:166.332
- Hs Code.:2930909090
- European Community (EC) Number:219-012-4
- DSSTox Substance ID:DTXSID50177711
- Nikkaji Number:J55.531B
- Wikidata:Q83048030
- Mol file:2314-49-0.mol
Synonyms:Diethyl trithiocarbonate;2314-49-0;Carbonotrithioic acid, diethyl ester;bis(ethylsulfanyl)methanethione;CARBONIC ACID, TRITHIO-, DIETHYL ESTER;EINECS 219-012-4;BRN 1745274;SCHEMBL35180;bis(ethylsulfanyl)-methanethione;C5H10S3;DTXSID50177711;Trithiocarbonic acid diethyl ester;Trithiocarbonic acid, diethyl ester;C5-H10-S3;AKOS006278479;LS-52139