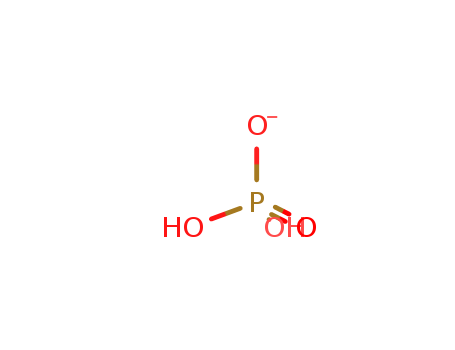
- Chemical Name:Dihydrogenphosphate
- CAS No.:14066-20-7
- Molecular Formula:H2O4 P
- Molecular Weight:96.9872
- Hs Code.:
- DSSTox Substance ID:DTXSID40930897
- Nikkaji Number:J238.533C,J2.449.279F,J997.145I,J3.155.004A
- Wikipedia:Dihydrogen phosphate ion,Dihydrogen_phosphate
- Wikidata:Q27093789,Q27110323
- Mol file:14066-20-7.mol
Synonyms:DIHYDROGEN PHOSPHATE;dihydrogenphosphate;DIHYDROGENPHOSPHATE ION;14066-20-7;dihydrogentetraoxophosphate(V);dihydrogentetraoxophosphate(1-);dihydroxidodioxidophosphate(1-);CHEBI:39745;dihydrogen(tetraoxidophosphate)(1-);H2PO4(-);(PO2(OH)2)(-);[PO2(OH)2](-);Oxylatophosphonic acid;H2O4P;Oxylatodihydroxyphosphine oxide;(Phosphoric acid dihydrogen)ion;H2PO4-;DTXSID40930897;H2-O4-P;BDBM50155534;DB02831;Q27093789;Q27110323