- Chemical Name:Bromine chloride
- CAS No.:13863-41-7
- Molecular Formula:BrCl
- Molecular Weight:115.357
- Hs Code.:
- European Community (EC) Number:237-601-4
- ICSC Number:1713
- UN Number:2901
- UNII:7G62XY5724
- DSSTox Substance ID:DTXSID4035259
- Nikkaji Number:J95.101C
- Wikipedia:Bromine chloride,Bromine monochloride,Bromine_monochloride
- Wikidata:Q421222
- Mol file:13863-41-7.mol
Synonyms:bromine chloride


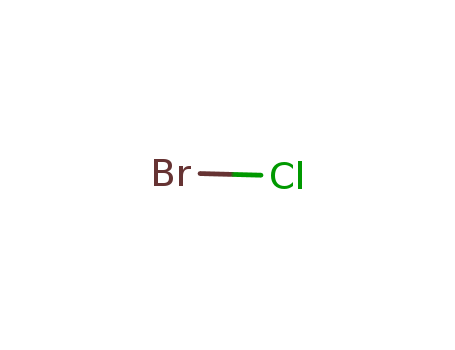

 F,
F, Xi
Xi


