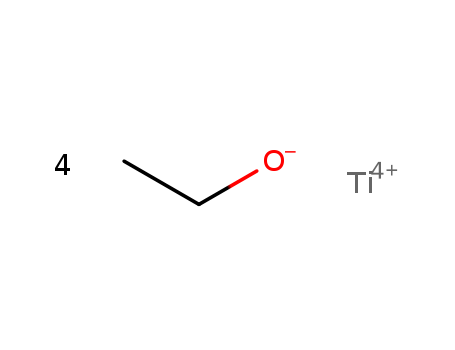
- Chemical Name:Tetraethoxytitanium
- CAS No.:3087-36-3
- Deprecated CAS:104814-62-2,260561-26-0,334497-44-8,426266-86-6,753456-54-1,97583-04-5
- Molecular Formula:C8H20O4Ti
- Molecular Weight:228.124
- Hs Code.:29051900
- European Community (EC) Number:221-410-8
- DSSTox Substance ID:DTXSID3044417
- Wikipedia:Titanium_ethoxide
- Mol file:3087-36-3.mol
Synonyms:Titanium(IV) ethoxide;3087-36-3;tetraethoxytitanium;Tetraethyl orthotitanate;Tetraethyl titanate;ethanol;titanium;Titanium(IV)ethoxide;Titanium, tetraethoxy-;MFCD00009071;Titanium (IV) ethoxide;Titanium (IV) Ethoxide (~80per cent, contain 20per cent Isopropoxide);SCHEMBL35278;DTXSID3044417;AKOS025213456;Titanium(IV) ethoxide, technical grade;Titanium(IV) ethoxide (99.99%-Ti);753456-54-1;BP-20364;S12401;EN300-7353571;F0001-0940;Titanium ethoxide, contains 10-15% Titanium(IV) isopropoxide;Tetraethyl Orthotitanate (contains 35% Tetraisopropyl Orthotitanate at maximum)


 Xi
Xi


