10.1021/jo01225a006
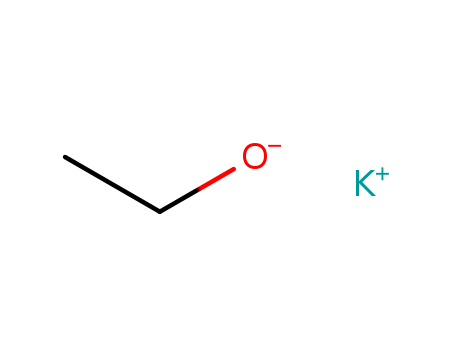
The study investigates the preparation, alkylation, and acylation of salts derived from phenylnitroacetonitrile, a secondary nitro compound. The researchers used various chemicals, including potassium ethoxide, d- and I-2-octyl nitrate, and benzyl cyanide, to synthesize the salts. They explored the optical properties of these salts and found that they were optically inactive, suggesting a conjugated aci-structure. In the alkylation process, the silver salt of phenylnitroacetonitrile reacted with methyl iodide to produce a nitronic ester, which was confirmed through catalytic reduction to phenylethylenediamine. The acylation process involved treating the salts with benzoyl chloride, resulting in a benzoyl derivative whose structure was established as an oxygen acylated compound through reduction to benzoic acid and phenylethylenediamine. The study provides insights into the structural preferences and reactivity of these nitro compound salts.


 F,
F, C
C


