10.1021/jo301607a
The research focuses on the development of a convenient synthesis method for 1,2,3-thiadiazoles and 1,2,3-selenadiazoles, which are important heterocyclic compounds with broad pharmacological properties, including anticancer, antibacterial, and antifungal activities. The study utilizes an ionic liquid as a novel soluble support, offering a simple and efficient approach to synthesize these compounds. The methodology involves the synthesis of ionic liquid-supported sulfonyl hydrazine, which is then reacted with various ketones and 1,2-diketones to form ionic liquid-supported hydrazones. These hydrazones are subsequently converted to 1,2,3-thiadiazoles in the presence of thionyl chloride, while 1,2,3-selenadiazoles are obtained by reacting the hydrazones with selenium dioxide in acetonitrile. The research concludes that this approach offers several advantages, such as ease of workup, simple reaction conditions, and high purity of the products. The chemicals used in the process include 1-methylimidazole, 1,4-butane sultone, trifluoromethane sulfonic acid (TfOH), thionyl chloride, hydrazine hydrate, and various ketones and 1,2-diketones, as well as selenium dioxide for the synthesis of selenadiazoles.
10.1002/jhet.5570350116
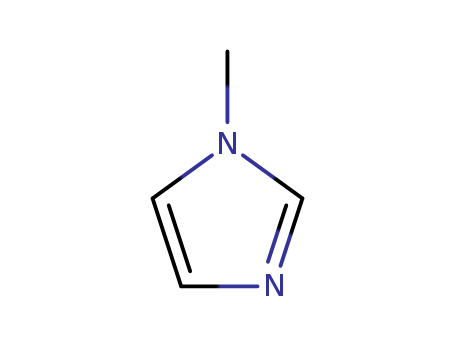
The research focuses on the synthesis of aryl o-(1-methyl-5-imidazolyl and 1H-5-tetrazolyl)alkyl ketones, which are complex organic compounds with potential applications in medicinal chemistry. The study outlines various approaches to synthesize these ketones, including the use of lithiation-substitution reactions, Claisen-Schmidt condensations, and the van Leusen synthesis of imidazoles. The purpose of the research was to develop efficient methods for the preparation of these compounds, which involve intricate steps such as lithiation of imidazoles, substitution with various reagents, and hydrolysis of protective groups. The conclusions of the research detail the successful synthesis of the target ketones, along with the challenges encountered in the process, such as the instability of certain intermediates and the complexity of the synthetic pathways. Chemicals used in the process include 1-methylimidazole, 1,2-dimethylimidazole, α-iodoalkyl ketals, 2,4-dichlorophenyl and 4-chlorophenyl ketones, as well as various protecting groups and reagents for the lithiation and substitution reactions.
10.1039/c39890000997
T. Sudhakar Rao and Colin B. Reese describe a new method for synthesizing 2,3'-anhydrothymidine (3), a key intermediate in the production of the antiretroviral drug 3'-azido-3'-deoxythymidine (AZT). The authors discovered that heating thymidine (2) with an excess of diphenyl sulphite in dimethylacetamide solution in the presence of a catalytic amount of 1-methylimidazole at 156°C for 45 minutes yields 2,3'-anhydrothymidine (3) in approximately 65% yield. This method is advantageous over previous procedures, such as the use of 2-chloro-1-diethylamino-1,1,2-trifluoroethane, due to the greater accessibility of diphenyl sulphite. The synthesized 2,3'-anhydrothymidine (3) can then be converted into AZT by reacting with lithium azide, achieving a 71% yield of AZT. This streamlined synthesis offers a more efficient route for the production of AZT, which is crucial for the treatment of AIDS patients.
10.1021/ja00212a030
The study presented in the document investigates the biomimetic oxidation of 1,2-diols using molecular oxygen in the presence of iron-porphyrin catalysts, mimicking the function of metal-containing oxidases and oxygenases found in biological systems. The researchers utilized a catalytic system comprising an iron-porphyrin complex, 1-benzyl-3-carbamoyl-1,4-dihydropyridine (BNAH), and molecular oxygen to selectively cleave the carbon-carbon bonds of aryl-substituted ethane-1,2-diols at room temperature, producing aldehydes or ketones as the main oxidation products. The reaction rates were influenced by the steric hindrance of substituents in both the catalysts and diols, and no significant differences in reactivities were observed between the two stereoisomers (meso and dl) of the diols. The study provides insights into the mechanism of the diol cleavage reaction, which involves the initial binding of the diol to the active catalyst forming an intermediate complex, followed by a rate-determining breakdown step in the catalytic cycle. The findings have implications for understanding the activation of molecular oxygen and oxygen atom transfer to organic substrates, processes that are crucial for cytochrome P-450 in biological systems.
10.1021/ol063017g
The study investigates a novel tandem reaction involving benzyne and N-substituted imidazoles to synthesize aryl amines containing anthracene under mild conditions. Benzyne, generated from o-trimethylsilyl aryltriflates, reacts with various N-substituted imidazoles, such as N-methylimidazole, N-ethylimidazole, and N-benzylimidazole, to form aryl amines with anthracene in a one-pot process. The reaction is believed to proceed via a tandem mechanism involving a Diels-Alder reaction and an intermolecular nucleophilic coupling reaction. The ratio of benzyne to imidazole is crucial for the reaction direction, and the optimized conditions include a 1:1 ratio at 50 °C in acetonitrile solvent for 12 hours. The study provides a transition-metal-free method to construct aryl amines containing anthracene, which are important building blocks in natural products and functional materials.


 C,
C, Xn,
Xn, F
F


