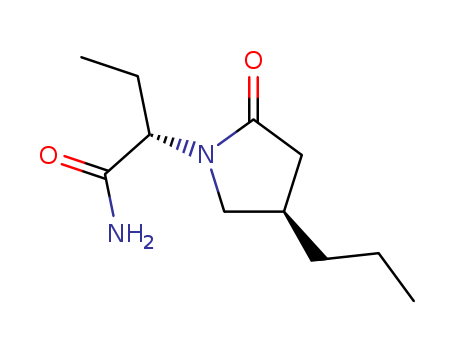
- Chemical Name:Brivaracetam
- CAS No.:357336-20-0
- Molecular Formula:C11H20 N2 O2
- Molecular Weight:212.292
- Hs Code.:2933990090
- European Community (EC) Number:801-184-2
- UNII:U863JGG2IA
- DSSTox Substance ID:DTXSID00905081
- Nikkaji Number:J2.411.659J
- Wikipedia:Brivaracetam
- Wikidata:Q408099
- NCI Thesaurus Code:C65270
- RXCUI:1739745
- Pharos Ligand ID:BRWYT3F6AUL5
- Metabolomics Workbench ID:149511
- ChEMBL ID:CHEMBL607400
- Mol file:357336-20-0.mol
Synonyms:(2S)-2-((4R)-2-oxo-4-propylpyrrolidin-1-yl)butanamide;1-pyrrolidineacetamide, alpha-ethyl-2-oxo-4-propyl-, (alphaS,4R)-;2-(2-oxo-4-propylpyrrolidin-1-yl)butanamide;brivaracetam;Briviact;UCB 34714;UCB-34714;UCB34714