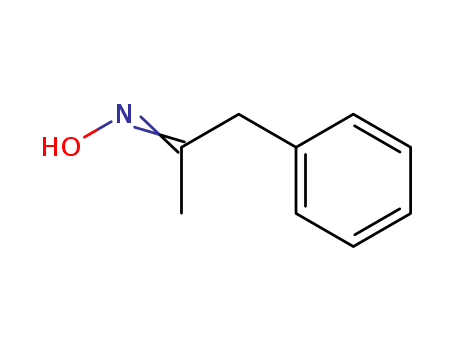
- Chemical Name:Phenylacetone oxime
- CAS No.:13213-36-0
- Molecular Formula:C9H11 N O
- Molecular Weight:149.192
- Hs Code.:2928000090
- Nikkaji Number:J1.099.313J
- NSC Number:14435
- Mol file:13213-36-0.mol
Synonyms:phenylacetone oxime;phenylacetone oxime, (E)-isomer;phenylacetone oxime, (Z)-isomer