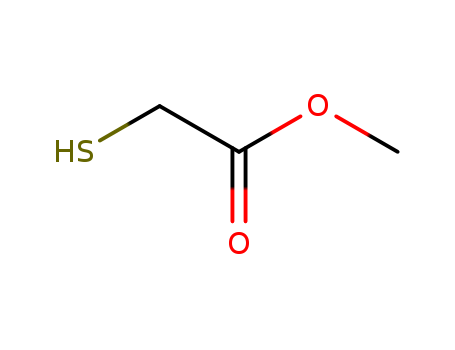
- Chemical Name:Methyl thioglycolate
- CAS No.:2365-48-2
- Deprecated CAS:64807-90-5,1629585-98-3
- Molecular Formula:C3H6 O2 S
- Molecular Weight:106.145
- Hs Code.:29309070
- European Community (EC) Number:219-121-7
- NSC Number:75117
- UNII:O1608LA9EL
- DSSTox Substance ID:DTXSID0033673
- Nikkaji Number:J54.943F
- Wikidata:Q27285190
- ChEMBL ID:CHEMBL1341329
- Mol file:2365-48-2.mol
Synonyms:methyl mercaptoacetate;methyl thioglycolate;methyl thioglycolate, sodium salt;thioglycolic acid methyl ester