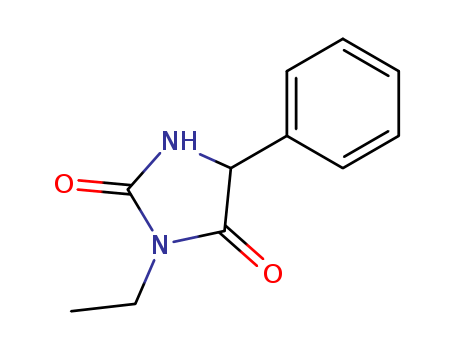
- Chemical Name:Ethotoin
- CAS No.:86-35-1
- Molecular Formula:C11H12 N2 O2
- Molecular Weight:204.228
- Hs Code.:2933210000
- European Community (EC) Number:201-665-1
- NSC Number:760074
- UNII:46QG38NC4U
- DSSTox Substance ID:DTXSID6023020
- Nikkaji Number:J3.894F
- Wikipedia:Ethotoin
- Wikidata:Q4533122
- NCI Thesaurus Code:C47524
- Pharos Ligand ID:YRK52NMJFWP6
- Metabolomics Workbench ID:144288
- ChEMBL ID:CHEMBL1095
- Mol file:86-35-1.mol
Synonyms:ethotoin;Peganone