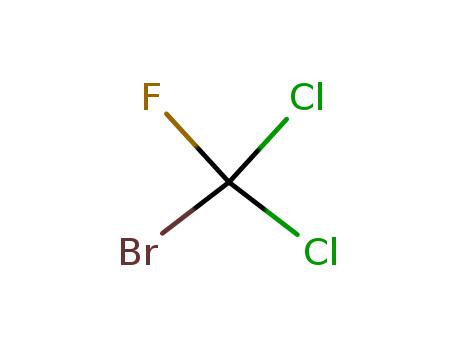
- Chemical Name:bromodichlorofluoromethane
- CAS No.:353-58-2
- Molecular Formula:CBrCl2F
- Molecular Weight:181.819
- Hs Code.:2903799090
- European Community (EC) Number:206-536-3
- UNII:IHD7R7A3OI
- DSSTox Substance ID:DTXSID2074291
- Nikkaji Number:J101.902C
- Wikidata:Q27280730
- Mol file:353-58-2.mol
Synonyms:Bromo(dichloro)fluoromethane;