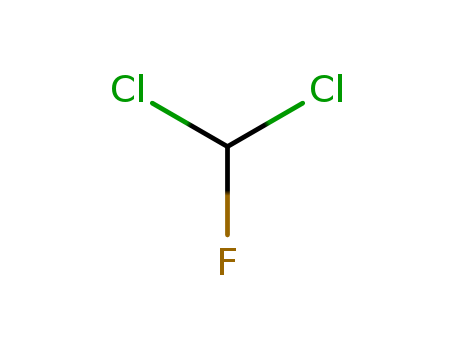
- Chemical Name:Dichlorofluoromethane
- CAS No.:75-43-4
- Deprecated CAS:39289-28-6
- Molecular Formula:CHCl2F
- Molecular Weight:102.923
- Hs Code.:
- European Community (EC) Number:200-869-8
- ICSC Number:1106
- UN Number:1029
- UNII:7GAO4CRJ0B
- DSSTox Substance ID:DTXSID7052498
- Nikkaji Number:J1.454K
- Wikipedia:Dichlorofluoromethane
- Wikidata:Q418163,Q83038271
- NCI Thesaurus Code:C77459
- ChEMBL ID:CHEMBL116813
- Mol file:75-43-4.mol
Synonyms:dichlorofluoromethane;fluorodichloromethane;Freon 21


 N
N


