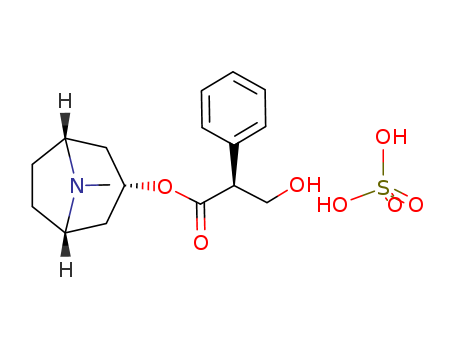
- Chemical Name:Hyoscyamine sulfate
- CAS No.:6835-16-1
- Molecular Formula:C34H52N2O12S
- Molecular Weight:387.454
- Hs Code.:2939800000
- Mol file:6835-16-1.mol
Synonyms:[3(S)-ENDO]-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL ESTER, ALPHA-(HYDROXYMETHYL)-BENZENEACETIC ACID SULFATE DIHYDRATE;HYOSCYAMINE SULFATE DIHYDRATE;HYOSCYAMINE SULPHATE;HYOSCYAMINE SULFATE;BENZENEACETIC ACID, ALPHA-(HYDROXYMETHYL)-, 8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL ESTER, [3(S)-ENDO]-, SULFATE (2:1), DIHYDRATE;HYOSCYAMINESULFATE,USP;HOSCYAMINE SULFATE USP