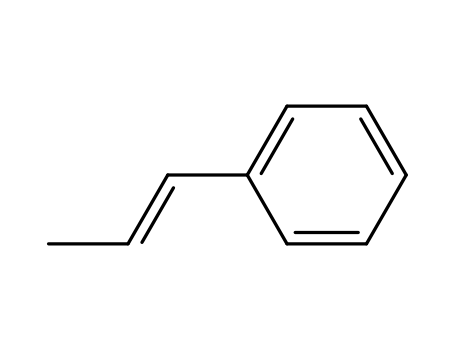
- Chemical Name:trans-beta-Methylstyrene
- CAS No.:873-66-5
- Molecular Formula:C9H10
- Molecular Weight:118.178
- Hs Code.:29029000
- European Community (EC) Number:212-848-0,211-287-9
- ICSC Number:0736
- NSC Number:73958,65591
- UN Number:2618
- UNII:A5S9N1785O
- DSSTox Substance ID:DTXSID2060919,DTXSID101026543
- Nikkaji Number:J2.100H,J2.513.017K,J54.347K
- Wikipedia:Trans-Propenylbenzene
- Wikidata:Q2020228
- Metabolomics Workbench ID:140309
- ChEMBL ID:CHEMBL506013
- Mol file:873-66-5.mol
Synonyms:1-phenylpropene;beta-methylstyrene;isoallylbenzene;trans-phenylpropene