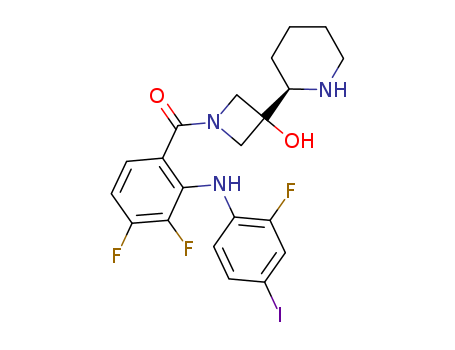
- Chemical Name:Cobimetinib
- CAS No.:934660-93-2
- Deprecated CAS:1029872-29-4
- Molecular Formula:C21H21F3IN3O2
- Molecular Weight:531.316
- Hs Code.:29333990
- UNII:ER29L26N1X
- ChEMBL ID:CHEMBL2146883
- DSSTox Substance ID:DTXSID60239435
- Metabolomics Workbench ID:149423
- NCI Thesaurus Code:C68923
- Nikkaji Number:J3.423.737I
- Pharos Ligand ID:8UDBJA4F9RNN
- RXCUI:1722365
- Wikidata:Q15708292
- Wikipedia:Cobimetinib
- Mol file:934660-93-2.mol
Synonyms:(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)(3-hydroxy-3-(piperidin-2-yl)azetidin-1-yl)methanone;cobimetinib;Cotellic;GDC-0973;XL518