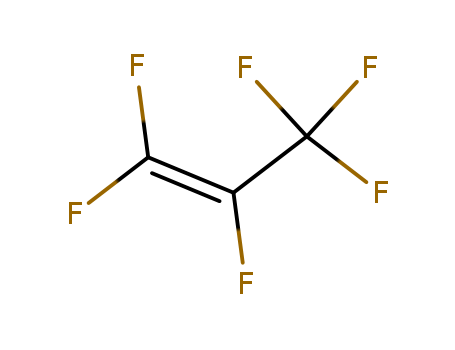
- Chemical Name:Hexafluoropropene
- CAS No.:116-15-4
- Molecular Formula:C3F6
- Molecular Weight:150.023
- Hs Code.:2903391000
- European Community (EC) Number:204-127-4
- UN Number:1858
- UNII:TRW23XOS20
- DSSTox Substance ID:DTXSID2026949
- Nikkaji Number:J35.205E
- Wikipedia:Hexafluoropropylene
- Wikidata:Q417982
- ChEMBL ID:CHEMBL4644489
- Mol file:116-15-4.mol
Synonyms:hexafluoropropene



 Xn:Harmful;
Xn:Harmful;

