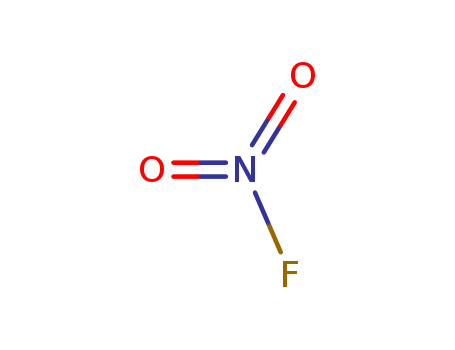
- Chemical Name:Nitryl fluoride
- CAS No.:10022-50-1
- Molecular Formula:FN O2
- Molecular Weight:65.0051
- Hs Code.:
- European Community (EC) Number:233-021-0
- UNII:DAT2I9R64A
- DSSTox Substance ID:DTXSID00143027
- Wikipedia:Nitryl fluoride
- Wikidata:Q2613976
- Mol file:10022-50-1.mol
Synonyms:Nitryl fluoride;10022-50-1;NO2F;UNII-DAT2I9R64A;DAT2I9R64A;EINECS 233-021-0;nitric acid fluoride;FNO2;NITRYL FLUORIDE [MI];F-N-O2;FLUORINE NITRITE (FNO2);DTXSID00143027;NITRYL FLUORIDE ((NO2)F);Q2613976