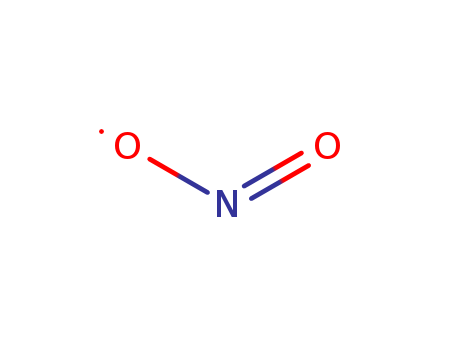
- Chemical Name:Nitrogen Dioxide
- CAS No.:10102-44-0
- Deprecated CAS:119990-11-3,127999-62-6,50443-93-1,56003-83-9,66252-28-6,78246-05-6
- Molecular Formula:NO2
- Molecular Weight:46.0055
- Hs Code.:
- European Community (EC) Number:233-272-6
- ICSC Number:0930
- UN Number:1067
- DSSTox Substance ID:DTXSID7020974
- Nikkaji Number:J2.226.857K
- Wikipedia:Nitrogen dioxide,Nitrogen_dioxide
- NCI Thesaurus Code:C29843
- Mol file:10102-44-0.mol
Synonyms:Dioxide, Nitrogen;Nitrogen Dioxide;Nitrogen Peroxide;Peroxide, Nitrogen