- Chemical Name:Benzene-d6
- CAS No.:1076-43-3
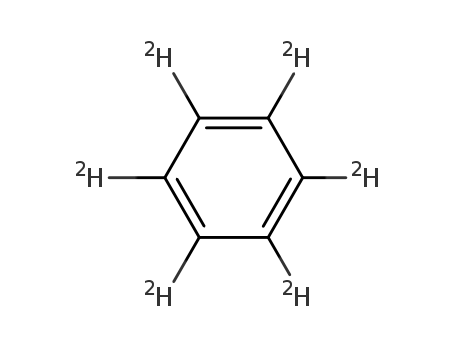
- Molecular Formula:C6D6
- Molecular Weight:84.066
- Hs Code.:29022000
- European Community (EC) Number:214-061-8
- DSSTox Substance ID:DTXSID7037769
- Nikkaji Number:J95.385G
- Wikipedia:Deuterated_benzene
- Wikidata:Q1101314
- Mol file:1076-43-3.mol
Synonyms:Benzene-d6;1076-43-3;Perdeuterobenzene;Hexadeuterobenzene;1,2,3,4,5,6-hexadeuteriobenzene;Benzene D6;hexadeuteriobenzene;(2H6)Benzene;Benzene-1,2,3,4,5,6-d6;C6D6;EINECS 214-061-8;Benzene-d6, 99.6 atom % D;MFCD00003010;Deuterated benzene;Benzene D6 2000 microg/mL in Methanol;Benzene-d6, "100%", 99.96 atom % D;benzol-d6;d6 -benzene;D6-Benzene;[D6]benzene;Benzene (D6);Benzene-D6 >99%;Benzene D6 >99.96%;Benzene-d6, 99 atom % D;DTXSID7037769;CHEBI:193039;(Deuterated benzene);C6-D6;CS-B1393;AKOS015888202;AS-75441;Benzene-d6, 99.6 atom % D, anhydrous;Benzene-D6 >99% (+0.03% TMS);B0840;B3008;Benzene-d6, anhydrous, >=99.6 atom % D;Benzene-D6 >99.5% (+0.03% TMS);F11732;J-001990;Q1101314;Benzene-d6, 100.0 atom % D, >=99.96 atom % D;Benzene-d6, 99.6 atom % D, contains 1 % (v/v) TMS;Benzene-d6, 99.6 atom % D, contains 0.03 % (v/v) TMS;Benzene-d6, "100%", 99.96 atom % D, contains 0.03 % (v/v) TMS


 F,
F, T
T

