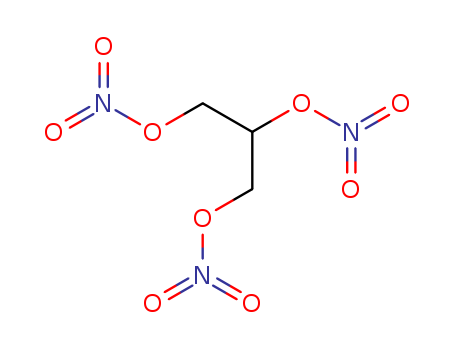
- Chemical Name:Nitroglycerin
- CAS No.:55-63-0
- Deprecated CAS:105469-31-6,80066-48-4,8013-23-8,9010-02-0,100292-13-5,100292-13-5,8013-23-8,9010-02-0
- Molecular Formula:C3H5N3O9
- Molecular Weight:227.087
- Hs Code.:2920900002
- European Community (EC) Number:200-240-8
- ICSC Number:0186
- UN Number:0144,0143
- UNII:G59M7S0WS3
- DSSTox Substance ID:DTXSID1021407
- Wikipedia:Nitroglycerin
- Wikidata:Q162867
- NCI Thesaurus Code:C29294
- RXCUI:4917
- Pharos Ligand ID:8JZKV6G7YNVM
- Metabolomics Workbench ID:43033
- ChEMBL ID:CHEMBL730
- Mol file:55-63-0.mol
Synonyms:Anginine;Dynamite;Gilustenon;Glyceryl Trinitrate;Nitrangin;Nitro Bid;Nitro Dur;Nitro-Bid;Nitro-Dur;NitroBid;Nitrocard;Nitroderm;Nitroderm TTS;NitroDur;Nitroglycerin;Nitroglyn;Nitrol;Nitrolan;Nitrong;Nitrospan;Nitrostat;Perlinganit;Susadrin;Sustac;Sustak;Sustonit;Transderm Nitro;Tridil;Trinitrate, Glyceryl;Trinitrin;Trinitrolong


 E,
E,  T+,
T+,  N
N


