10.1016/j.tet.2016.12.035
The research focuses on the synthesis of carotenoid-monosaccharide conjugates through an azide-alkyne click reaction using bis-triphenylphosphano-copper(I)-butyrate (C3H7COOCu(PPh3)2) as a catalyst. The study involves coupling carotenoid pentynoates with protected and unprotected sugar azides to form amphipathic carotenoid-sugar derivatives. The reactants include hydroxy carotenoids, 4-pentynoic acid, various sugar azides, and the copper catalyst. The synthesized compounds were characterized using various analytical techniques such as NMR spectroscopy, mass spectrometry (MALDI-TOF), infrared spectroscopy, and elemental analysis. The experiments resulted in the formation of target triazoles with good to excellent yields, offering a new approach for synthesizing biologically interesting molecules with potential applications in enhancing the stability of cell membranes and acting as antioxidants.
10.1016/j.tetlet.2006.06.129
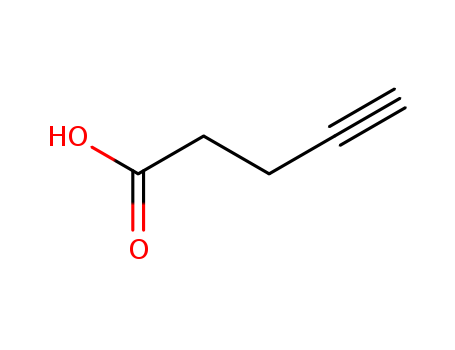
The research explores an efficient and stereoselective method for converting x-acetylenic acids into enol lactones using gold catalysis. The study investigates the cyclization of acetylenic acids mediated by gold species, focusing on finding the optimal conditions for this transformation. Key chemicals involved in the research include gold chloride (AuCl), potassium carbonate (K2CO3), and various acetylenic acids such as 4-pentynoic acid, 5-hexynoic acid, and 6-heptynoic acid. The researchers discovered that the combination of AuCl and K2CO3 in solvents like acetonitrile or tetrahydrofuran (THF) effectively promoted the cyclization, yielding the desired enol lactones with high selectivity for the exo-dig product. The study also examines the influence of different substituents on the acetylenic acids and their impact on the reaction outcomes, highlighting the role of these chemicals in achieving the desired products with specific stereochemistry.


 C
C

