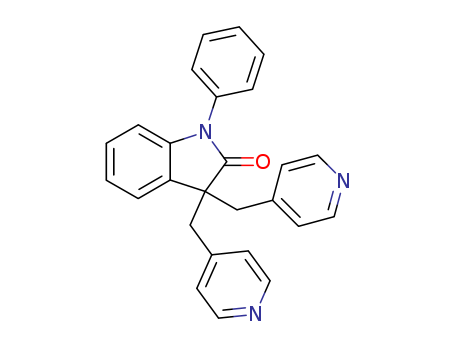
- Chemical Name:Linopirdine
- CAS No.:105431-72-9
- Molecular Formula:C26H21 N3 O
- Molecular Weight:391.472
- Hs Code.:2933790090
- UNII:I5TB3NZ94T
- DSSTox Substance ID:DTXSID6045163
- Nikkaji Number:J399.573I
- Wikipedia:Linopirdine
- Wikidata:Q6554730
- NCI Thesaurus Code:C82306
- Pharos Ligand ID:D5LDVLDUNMFY
- Metabolomics Workbench ID:67466
- ChEMBL ID:CHEMBL319111
- Mol file:105431-72-9.mol
Synonyms:3,3-bis(4-pyridylmethyl)-1-phenylindolin-2-one;3,3-dipyridylmethyl-1-phenyl-2-indolinone;DuP 996;DuP-996;linopirdine