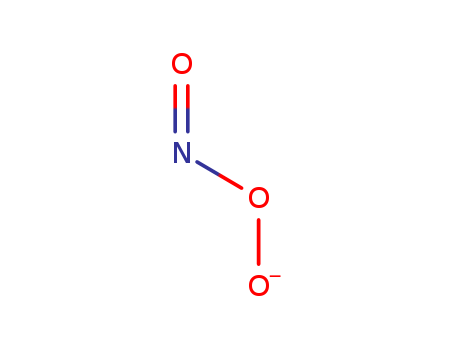
- Chemical Name:Oxido nitrite
- CAS No.:19059-14-4
- Molecular Formula:NO3
- Molecular Weight:62.0049
- Hs Code.:
- UNII:UR67NH4U77
- DSSTox Substance ID:DTXSID10172540
- Nikkaji Number:J2.724.771G
- Wikipedia:Peroxynitrite
- Wikidata:Q419130
- Mol file:19059-14-4.mol
Synonyms:oxido nitrite;peroxynitrite ion;19059-14-4;Oxoperoxonitrate(1-);UR67NH4U77;Peroxynitrite (8CI,9CI);Peroxonitrous acid anion;ONOO-;oxidoperoxidonitrate(1-);UNII-UR67NH4U77;Peroxynitrosyl (NO3) (6CI);CHEBI:25941;DTXSID10172540;[NO(OO)](-);LS-102485;Q419130