10.3184/030823408X360355
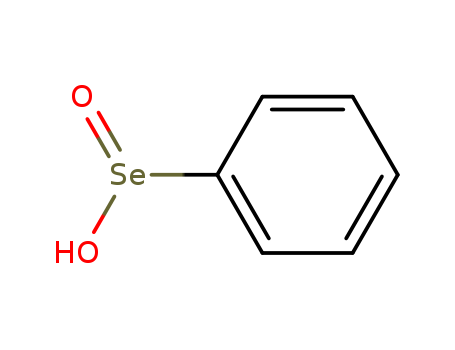
The research describes a novel and efficient method for the one-pot synthesis of aryl vinyl ethers using β-phenylselenoethanol as a reagent. The purpose of the study was to develop a more experimentally simple and efficient methodology for the preparation of aryl vinyl ethers, which are key intermediates in various synthetic applications and polymeric materials. The researchers achieved this through a two-step process involving the Mitsunobu reaction of β-phenylselenoethanol with phenols, followed by oxidation-elimination with 30% hydrogen peroxide. The method concluded with good yields (85–90%) and had the advantages of mild reaction conditions and convenient manipulation. Key chemicals used in the process included β-phenylselenoethanol, phenols, diphenyl diselenide, sodium hydride, HMPA, 2-chloroethanol, and various phenolic substrates with different substituents. The study also reported the recovery of diphenyl diselenide in a 60% yield through the addition of hydrazine monohydrate to the aqueous extract containing benzeneseleninic acid.


 T;
T;  N
N


