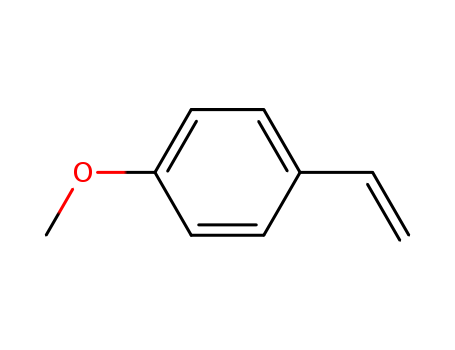
- Chemical Name:4-Methoxystyrene
- CAS No.:637-69-4
- Molecular Formula:C9H10O
- Molecular Weight:134.178
- Hs Code.:29093090
- European Community (EC) Number:211-298-9
- NSC Number:408326,42171
- UNII:2ISH8T4A6E
- DSSTox Substance ID:DTXSID7073222
- Nikkaji Number:J43.389F
- Wikipedia:4-Vinylanisole
- Wikidata:Q27254796
- Mol file:637-69-4.mol
Synonyms:Anisole,p-vinyl- (6CI,8CI);(4-Methoxyphenyl)ethene;(4-Methoxyphenyl)ethylene;1-Ethenyl-4-methoxybenzene;1-Methoxy-4-vinylbenzene;4-Methoxystyrene;4-Methoxyvinylbenzene;NSC 408326;NSC 42171;p-Anisylethylene;p-Methoxystyrene;p-Vinylanisole;