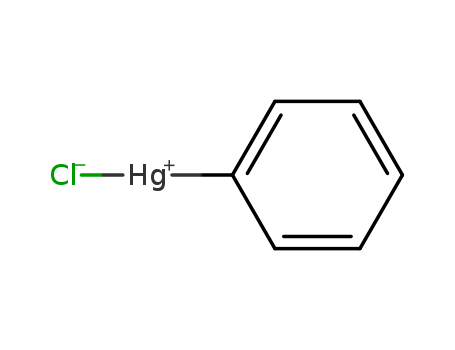
- Chemical Name:Phenylmercury(1+);chloride
- CAS No.:100-56-1
- Molecular Formula:C6H5 Cl Hg
- Molecular Weight:313.149
- Hs Code.:28439090
- UNII:X0R4ES0U7Z
- Mol file:100-56-1.mol
Synonyms:phenylmercury(1+);chloride;PHENYLMERCURIC CHLORIDE [MI];PHENYLMERCURIC CHLORIDE [HSDB];PHENYL MERCURIC CHLORIDE [INCI];P0176;SR-01000864592;SR-01000864592-2