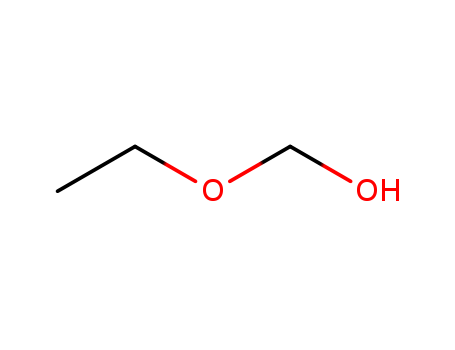
- Chemical Name:Ethoxymethanol
- CAS No.:10171-38-7
- Molecular Formula:C3H8 O2
- Molecular Weight:76.0953
- Hs Code.:2909499000
- European Community (EC) Number:233-446-1
- UNII:8BGJ5Z26A4
- DSSTox Substance ID:DTXSID5021592
- Nikkaji Number:J227.084F
- Wikidata:Q81983825
- Mol file:10171-38-7.mol
Synonyms:Ethoxymethanol;10171-38-7;Methanol, ethoxy-;EINECS 233-446-1;1-Ethoxy-1-hydroxymethane;2-ethoxymethanol;Methylene glycol ethyl ether;8BGJ5Z26A4;DTXSID5021592