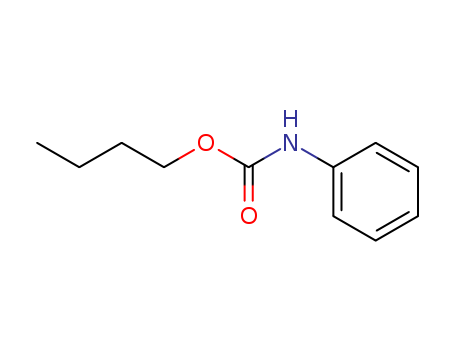
- Chemical Name:Butyl N-phenylcarbamate
- CAS No.:1538-74-5
- Molecular Formula:C11H15 N O2
- Molecular Weight:193.246
- Hs Code.:2924299090
- European Community (EC) Number:633-865-3
- NSC Number:29705
- DSSTox Substance ID:DTXSID60165422
- Nikkaji Number:J122.196E
- Wikidata:Q83034590
- Mol file:1538-74-5.mol
Synonyms:BUTYL N-PHENYLCARBAMATE;1538-74-5;Butyl phenylcarbamate;Butyl carbanilate;Carbanilic acid, butyl ester;Phenylcarbamic acid butyl ester;Butylphenylurethane;Butyl N-phenylurethane;Carbamic acid, phenyl-, butyl ester;Butylphenylcarbamate;n-butyl phenylcarbamate;Butyl phenylcarbamate #;Carbanilic acid, n-butyl ester;SCHEMBL3765824;Phenyl-carbamic acid butyl ester;DTXSID60165422;ZTIMKJROOYMUMU-UHFFFAOYSA-N;NSC29705;NSC 29705;NSC-29705;AKOS002953314;SB76753