10.1016/j.jorganchem.2009.06.036
The research investigates the synthesis, structural properties, and DNA-binding behavior of four new organotin(IV) carboxylates, namely [Bu2SnL2] (1), [Et2SnL2] (2), [Bu3SnL]n (3), and [Me3SnL]n (4), where L represents the 4-nitrophenylethanoates ligand. The purpose of this study is to provide insights into the drug-DNA interaction mechanism and potential drug design applications, particularly in the context of anticancer activity. The researchers synthesized these compounds using organotin(IV) precursors and characterized them through elemental analysis, FT-IR, multinuclear NMR, and X-ray single crystal analysis. Dibutyltin(IV) dichloride was used as a precursor for the synthesis of complex 1. The study found that the ligand coordinated to the organotin moiety via the COO group, with different coordination modes observed in the complexes. Cyclic voltammetry was employed to evaluate the electrochemical, kinetic, and thermodynamic parameters of these complexes interacting with DNA, revealing diffusion-controlled processes and binding constants indicating the order of binding strength as 2 < 1 < 4 < 3. Additionally, compounds 1 and 2 demonstrated anticancer activity against prostate cancer cell lines (PC-3), consistent with their voltammetric behavior. The research concludes that these organotin(IV) derivatives show potential as chemotherapeutic agents due to their intercalative interaction with DNA and their anticancer activity, suggesting further exploration for drug development.


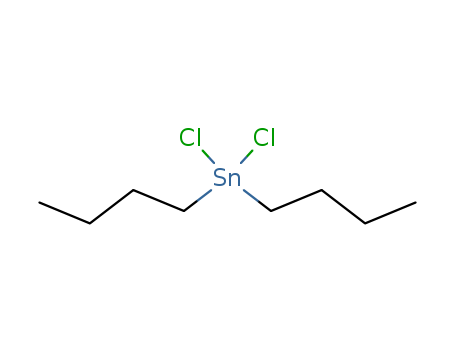
 T;
T;  N
N


