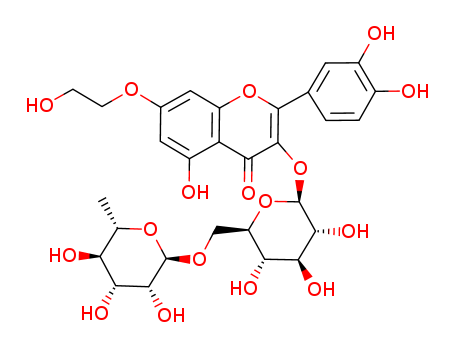
- Chemical Name:Monoxerutin
- CAS No.:23869-24-1
- Molecular Formula:C29H34 O17
- Molecular Weight:654.579
- Hs Code.:
- European Community (EC) Number:245-917-9
- UNII:EKF7043SBU
- DSSTox Substance ID:DTXSID70905139
- Nikkaji Number:J34.317J
- Wikipedia:Monoxerutin
- Wikidata:Q6902068
- NCI Thesaurus Code:C77402
- Metabolomics Workbench ID:144442
- ChEMBL ID:CHEMBL3527416
- Mol file:23869-24-1.mol
Synonyms:3',4',7-trihydroxyethylrutin;3',4',7-tris(O-(2- hydroxyethyl))rutin;oxerutin;Paroven;Posorutin;Relvene;Rhéoflux;Teboven;Troxérutine Mazal;troxerutin;Troxerutin-ratiopharm;Troxeven;Vastribil;Veinamitol;Veniten retard;Veno SL;Venorutin;venoruton;venoruton P4;Venotrulan Trox;vitamin P4;7-monohydroxyethylrutoside;monoHER