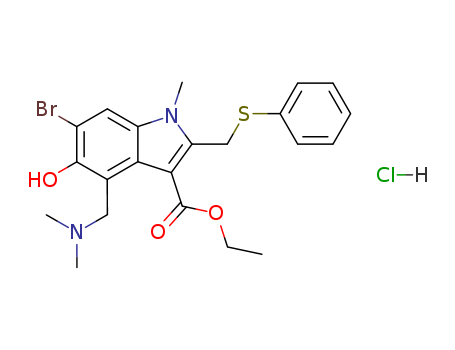
- Chemical Name:Arbidol Hydrochloride
- CAS No.:131707-23-8
- Molecular Formula:C22H26BrClN2O3S
- Molecular Weight:513.883
- Hs Code.:2933990090
- European Community (EC) Number:680-680-9
- UNII:8CXV4OU367
- Wikidata:Q4475505
- ChEMBL ID:CHEMBL5179853
- Mol file:131707-23-8.mol
Synonyms:1-methyl-2-((phenylthio)methyl)-3-carbethoxy-4-((dimethylamino)methyl)-5-hydroxy-6-bromoindole;1H-indole-3-carboxylic acid, 6-bromo-4-((dimethylamino)methyl)-5-hydroxy-1-methyl-2-((phenylthio)methyl)-, ethyl ester;arbidol;arbidole;umifenovir;umifenovir hydrochloride;umifenovir hydrochloride monohydrate;umifenovir sulfoxide