- Chemical Name:Sodium Bromide
- CAS No.:7647-15-6
- Deprecated CAS:59217-63-9
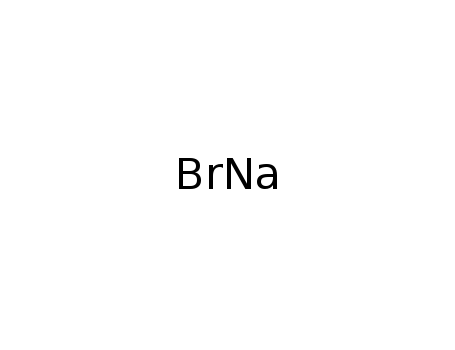
- Molecular Formula:NaBr
- Molecular Weight:102.894
- Hs Code.:2827.51
- European Community (EC) Number:231-599-9
- NSC Number:77384
- UNII:LC1V549NOM
- DSSTox Substance ID:DTXSID3034903
- Nikkaji Number:J44.028K
- Wikipedia:Sodium bromide
- Wikidata:Q15768
- NCI Thesaurus Code:C83922
- ChEMBL ID:CHEMBL1644694
- Mol file:7647-15-6.mol
Synonyms:sodium bromide;sodium bromide, 82Br-labeled


 Xi
Xi


