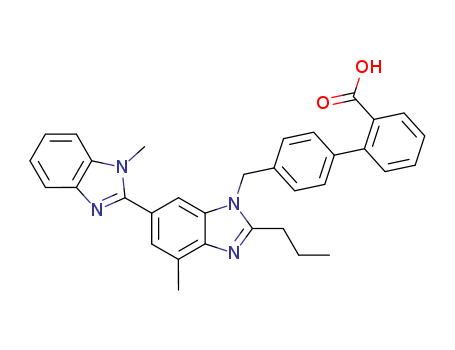
- Chemical Name:Telmisartan
- CAS No.:144701-48-4
- Molecular Formula:C33H30N4O2
- Molecular Weight:514.627
- Hs Code.:2933995300
- European Community (EC) Number:620-494-7
- NSC Number:759811
- UNII:U5SYW473RQ
- DSSTox Substance ID:DTXSID8023636
- Nikkaji Number:J556.167A
- Wikipedia:Telmisartan
- Wikidata:Q733186
- NCI Thesaurus Code:C47746
- RXCUI:73494
- Pharos Ligand ID:UXX5QY748TGQ
- Metabolomics Workbench ID:43221
- ChEMBL ID:CHEMBL1017
- Mol file:144701-48-4.mol
Synonyms:4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidazol)-1'-yl)methyl)-(1,1'-biphenyl)-2-carboxylic acid;BIBR 277;BIBR-277;Micardis;Pritor;telmisartan


 Xi
Xi


