- Chemical Name:1,1-Difluoroethane
- CAS No.:75-37-6
- Deprecated CAS:71281-71-5
- Molecular Formula:C2H4 F2
- Molecular Weight:66.0506
- Hs Code.:2903399090
- European Community (EC) Number:200-866-1,687-441-8
- ICSC Number:1729
- UN Number:1030
- UNII:0B1U8K2ME0
- DSSTox Substance ID:DTXSID0024050
- Nikkaji Number:J2.819C
- Wikipedia:1,1-Difluoroethane
- Wikidata:Q161285,Q83049583
- RXCUI:1305515
- ChEMBL ID:CHEMBL325493
- Mol file:75-37-6.mol
Synonyms:1,1-difluoroethane;ethylidene difluoride


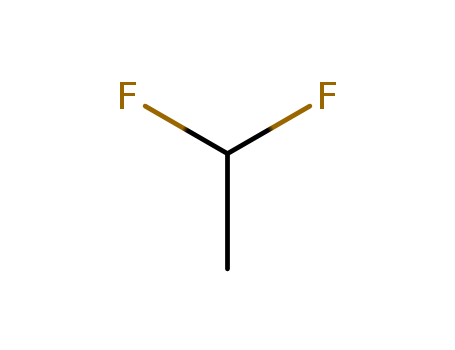
 F
F
 F:Flammable;
F:Flammable;

