10.1021/jo00171a021
The research explores a new method for synthesizing reserpine, a complex alkaloid with significant pharmaceutical importance. The study aims to develop a general synthetic methodology for constructing the hydroisoquinoline core structure found in reserpine, using amino-Claisen rearrangements of zwitterionic N-vinylisoquinuclidenes. Key chemicals used in this research include N-(indolylethyl)isoquinuclidenes, ethyl propiolate, and tert-butyl propiolate. The researchers demonstrated that these rearrangements can efficiently produce cis-fused hydroisoquinolines, which are crucial intermediates in the synthesis of reserpine. They also showed that the resulting hydroisoquinoline derivatives can undergo Wenkert cyclization to form pentacyclic systems resembling the natural product skeleton of reserpine. The study concludes that the combination of zwitterionic amino-Claisen rearrangements and Wenkert-type cyclizations offers a promising and efficient route for constructing the complex reserpine skeleton, with potential for further optimization and application in the synthesis of other Rauwolfia alkaloids.

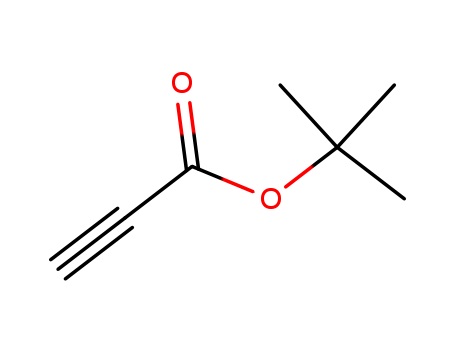

 Xi
Xi


