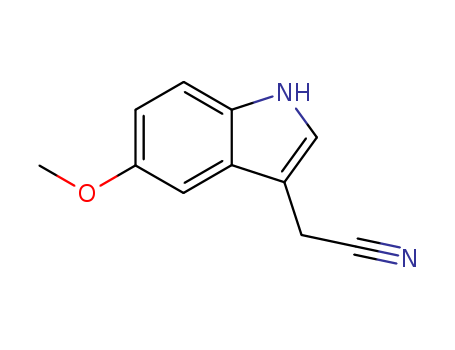
- Chemical Name:5-Methoxyindole-3-acetonitrile
- CAS No.:2436-17-1
- Molecular Formula:C11H10N2O
- Molecular Weight:186.213
- Hs Code.:2933990090
- European Community (EC) Number:800-083-0
- DSSTox Substance ID:DTXSID00393707
- Nikkaji Number:J1.650.104B
- Wikidata:Q72490039
- ChEMBL ID:CHEMBL2377609
- Mol file:2436-17-1.mol
Synonyms:5-Methoxyindole-3-acetonitrile;2436-17-1;2-(5-methoxy-1H-indol-3-yl)acetonitrile;5-Methoxy-3-indolylacetonitrile;(5-methoxy-1H-indol-3-yl)acetonitrile;2-(5-methoxy-1~{H}-indol-3-yl)ethanenitrile;3-(Cyanomethyl)-5-methoxyindole;AMY786;SCHEMBL2724551;CHEMBL2377609;DTXSID00393707;ZBQCXEREMRGOCO-UHFFFAOYSA-N;BCP26955;(5-methoxy-3-indolyl)-acetonitrile;5-Methoxy-1H-indole-3-acetonitrile;MFCD02094163;STL220633;AKOS005258779;5-Methoxy-3-indolylacetonitrile, 95%;(5-methoxy-1H-indol-3-yl)-acetonitrile;AC-27790;AS-11095;A5023;CS-0199379;FT-0650677;EN300-197564;M-3480;Z410711518;UOK


 Xi
Xi


