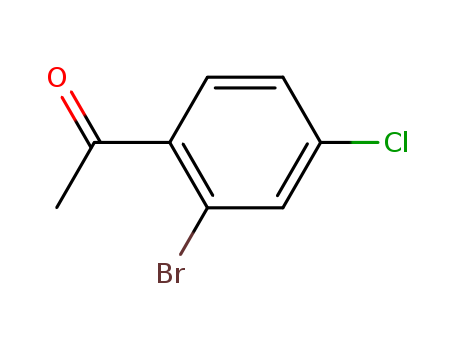
- Chemical Name:1-(2-Bromo-4-chlorophenyl)ethanone
- CAS No.:825-40-1
- Molecular Formula:C8H6 Br Cl O
- Molecular Weight:233.492
- Hs Code.:2914700090
- European Community (EC) Number:672-231-0
- DSSTox Substance ID:DTXSID80405736
- Wikidata:Q72452292
- Mol file:825-40-1.mol
Synonyms:1-(2-bromo-4-chlorophenyl)ethanone;825-40-1;2-Bromo-4-chloroacetophenone;4-acetyl-3-bromo-1-chlorobenzene;MFCD02683063;Ethanone, 1-(2-bromo-4-chlorophenyl)-;Ethanone,1-(2-bromo-4-chlorophenyl)-;SCHEMBL616449;DTXSID80405736;URBATMJMOGHOCE-UHFFFAOYSA-N;AMY16029;AKOS015891315;CS-W004840;AC-28641;DS-15914;SY057517;FT-0632996;A56001;2 inverted exclamation mark -Bromo-4 inverted exclamation mark -chloroacetophenone