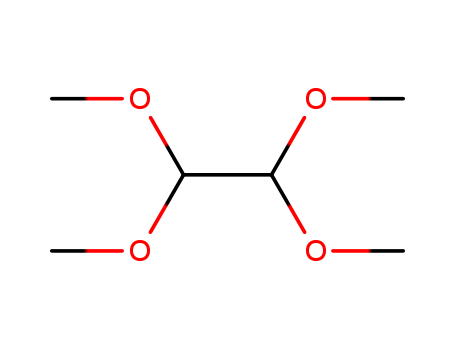
- Chemical Name:1,1,2,2-Tetramethoxyethane
- CAS No.:2517-44-4
- Molecular Formula:C6H14 O4
- Molecular Weight:150.175
- Hs Code.:2909199090
- European Community (EC) Number:463-280-7
- DSSTox Substance ID:DTXSID90339194
- Nikkaji Number:J121.207I
- Wikidata:Q82108044
- Mol file:2517-44-4.mol
Synonyms:1,1,2,2-Tetramethoxyethane;2517-44-4;Ethane, 1,1,2,2-tetramethoxy-;Tetramethoxy-ethane;1,1,2,2-tetramethoxyethan;Glyoxal bis(dimethyl acetal);1,1,2,2-tetramethoxy ethane;1,1,2,2-tetramethoxy-ethane;SCHEMBL2813868;DTXSID90339194;MFCD28361414;EN300-18521480